Text Solution
Verified by Experts
Topper's Solved these Questions
IS MATTER AROUND US PURE
LAKHMIR SINGH & MANJIT KAUR|Exercise Questions based on high order thinking skills (HOTS)|3 VideosIS MATTER AROUND US PURE
LAKHMIR SINGH & MANJIT KAUR|Exercise QUESTIONS BASED ON HIGH ORDER THINKING SKILLS (HOTS)|1 VideosIS MATTER AROUND US PURE
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT BOOK PAGE 24|1 VideosATOMS AND MOLECULES
LAKHMIR SINGH & MANJIT KAUR|Exercise Exercise|205 VideosMATTER IN OUR SURROUNDINGS
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT|26 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-IS MATTER AROUND US PURE -Exercise
- What Is Meant By Pure Substances
Text Solution
|
- List the points of differences between homogeneous and heterogenous mi...
Text Solution
|
- Differentiate between homogeneous and heterogeneous mixtures with exam...
Text Solution
|
- How are sol, solution and suspension different from each other ?
Text Solution
|
- To make a saturated solution, 36 g of sodium chloride is dissolved in ...
Text Solution
|
- How will you separate a mixture containing kerosene and petrol (differ...
Text Solution
|
- What type of mixtures are separted by the technique of craystallisatio...
Text Solution
|
- Classify the following as chemical or physical changes : ** cutting ...
Text Solution
|
- Try segregating the things around you as pure substances or mixtures.
Text Solution
|
- Which separation techniques will you apply for the separation of the f...
Text Solution
|
- Write the steps you would use for making tea. Use the words solution, ...
Text Solution
|
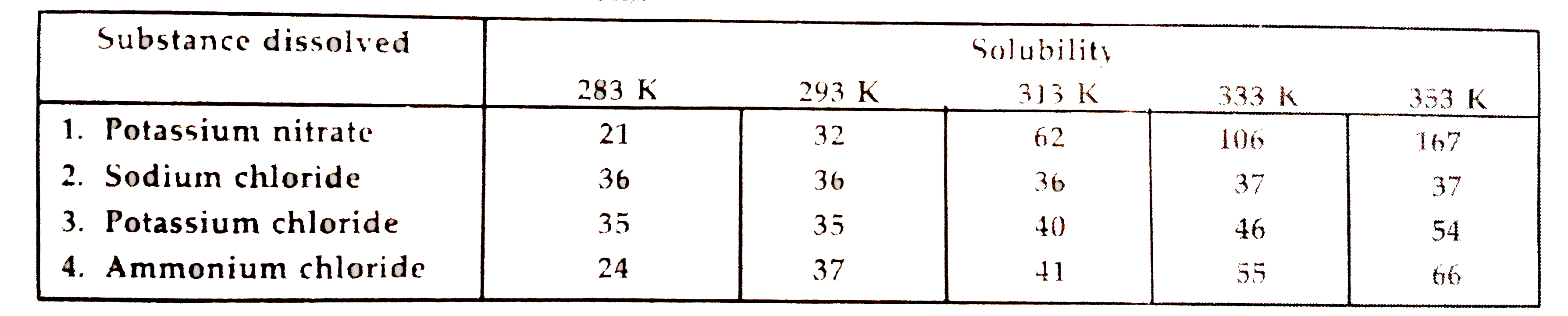
- Pargya tested the solubility of four different substances at different...
Text Solution
|
- Explain the following given examples: {:((a),"saturated solution",(b...
Text Solution
|
- Classify each of the following as a homogeneous or hetergeneous mixtur...
Text Solution
|
- How would you confirm that a colourless liquid given to you is pu...
Text Solution
|
- Which of the following materials fall in the category of a pure substa...
Text Solution
|
- Identify the solutions among the following mixtures. (a) Soil (b) ...
Text Solution
|
- Which of the following will show "Tyndall effect "? (a) Salt solutio...
Text Solution
|
- Classify the following into elements, compounds and mixtures. (a) So...
Text Solution
|
- Which of the following are chemical changes ? (a) Growth of a plant ...
Text Solution
|