A
B
C
D
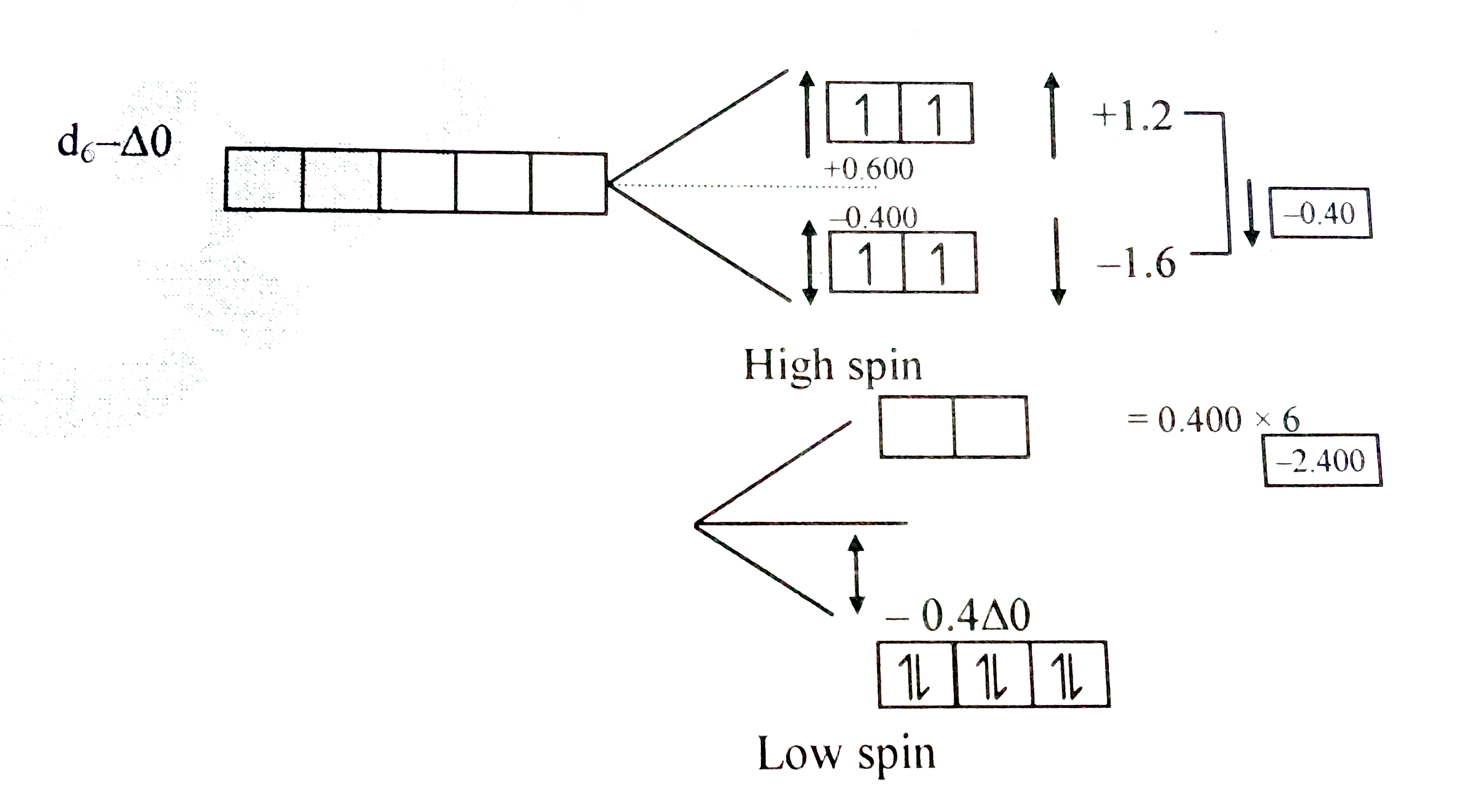
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The crystal field stabilization energies (CFSE) of high spin and low s...
Text Solution
|
- Crystal field stabilization energy for high spin d^4 octahedral comple...
Text Solution
|
- The crystal field stabilization energies (CFSE) of high spin and low s...
Text Solution
|
- Which one of the following high-spin complexes has the largest CFSE (C...
Text Solution
|
- Crystal field stabilization energy for high spin d^(4) octahedral comp...
Text Solution
|
- Crystal field stabilization energy for high spin d^(5) octahedral com...
Text Solution
|
- Crystal field stabilization energy for high spin d^(4) octahedral comp...
Text Solution
|
- The values of the crystal field stabilization energies for a high spin...
Text Solution
|
- Crystal field stabilization energy for high spin d^(5) octahedral com...
Text Solution
|