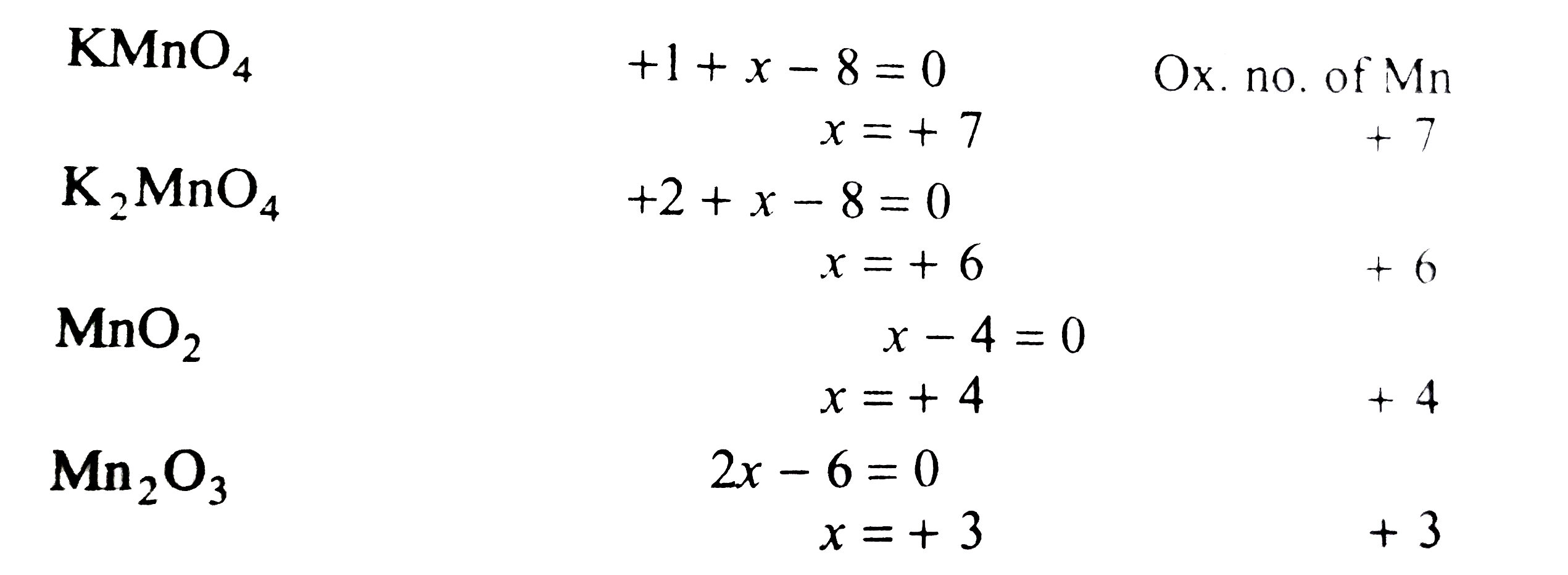
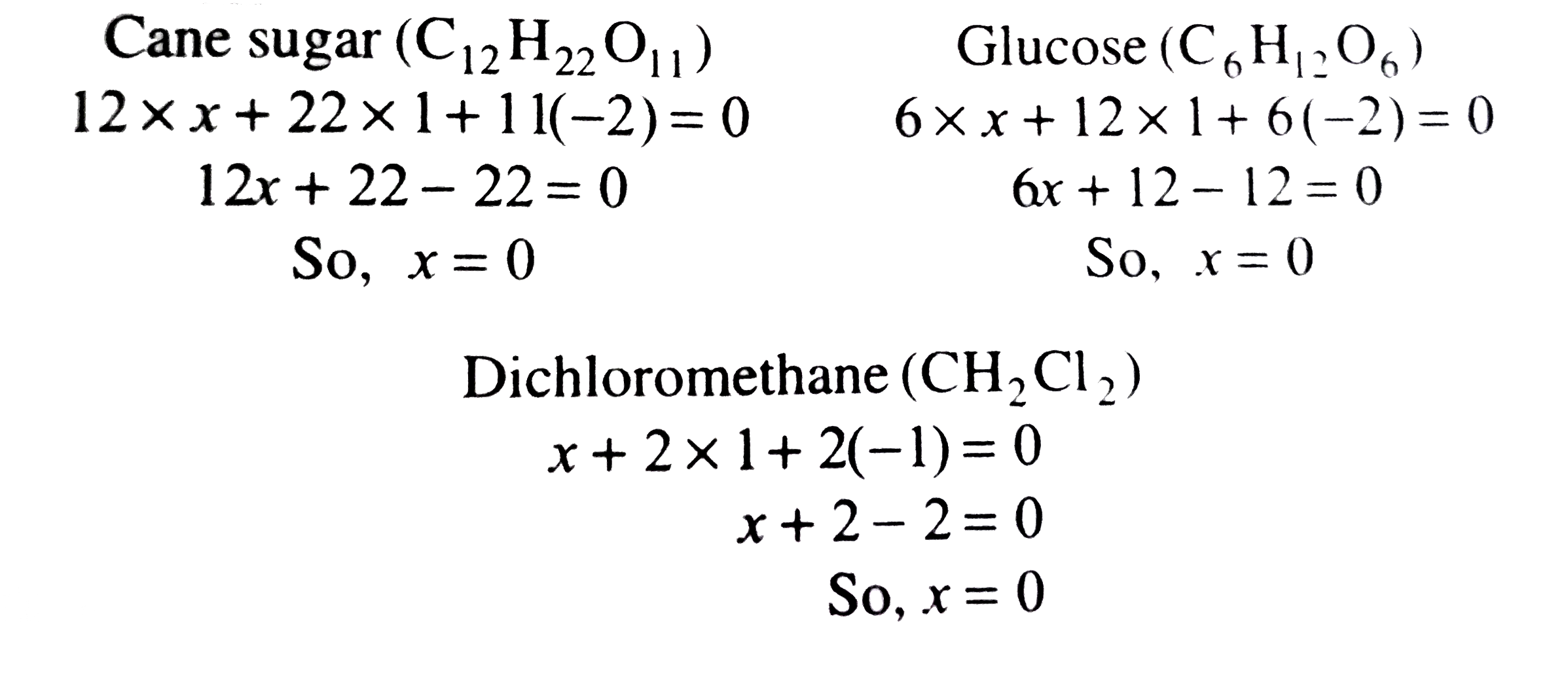Text Solution
Verified by Experts
Topper's Solved these Questions
OXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise ILLUSTRATION|5 VideosOXIDATION AND REDUCTION (REDOX REACTIONS)
OP TANDON|Exercise Practise Problems|5 VideosMISCELLANEOUS (TOPICS OF GENERAL INTEREST)
OP TANDON|Exercise SET-VI: Problems on graphical aptitude|53 VideosPOLYMERS
OP TANDON|Exercise PASSAGE 3|5 Videos
Similar Questions
Explore conceptually related problems
OP TANDON-OXIDATION AND REDUCTION (REDOX REACTIONS)-SECTION V
- Which compound amongst the following gas the highest oxidation number ...
Text Solution
|
- How many oxyanions among the following show disproportionation? ClO...
Text Solution
|
- The oxidation state of phosphorus in PO(4)^(3-),P(4)O(10) and P(2)O(7)...
Text Solution
|
- Oxidation state of phosphorus in cyclotrimetaphosphoric acid is
Text Solution
|