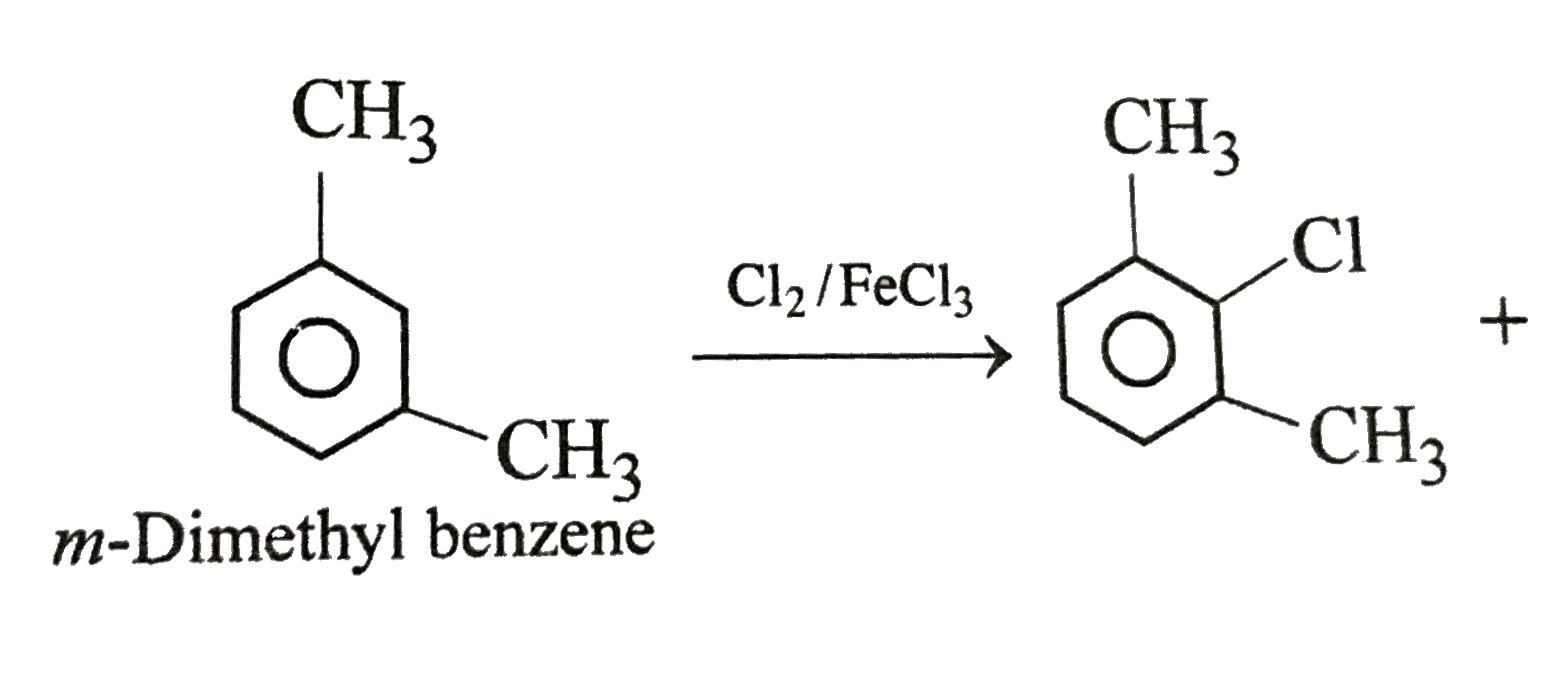
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-HYDROCARBONS-All Questions
- Benzene can undergo
Text Solution
|
- CaC(2)overset(H(2)O)toAoverset("Red tube hot")toBunderset(CH(3)Cl)over...
Text Solution
|
- The compound with molecular formula C(8)H(10) which will give only two...
Text Solution
|
- The decreasing order of acidic character among ethane(),ethene(II). Et...
Text Solution
|
- The alkene that will give the same product with HBr in the presence as...
Text Solution
|
- Chlorination of benzene in the presence of halogen carrier is an examp...
Text Solution
|
- In Kolbe's electrolysis method, electrolysis of aquesous solution of s...
Text Solution
|
- Ozonolysis of an organic compound gives formaldehyde as one of the pro...
Text Solution
|
- An acyclic hydrocarbon P, having molecular fromula C(6)H(10) gave acet...
Text Solution
|
- An acyclic hydrocarbon P, having molecular fromula C(6)H(10) gave acet...
Text Solution
|
- Which branched chain isomer of the hydrocarbon with molecular mass 72m...
Text Solution
|
- Hex-2-yne gives trans hex-2-ene on treatment with :
Text Solution
|
- In the following reaction H(3)C-underset(CH(3))underset(|)overset(CH...
Text Solution
|
- In allene (C(3)H(4)), the type(s) of hybridisation of the carbon atoms...
Text Solution
|
- Which of the following reagents will be able to distinguish between 1-...
Text Solution
|
- Some meta-directing substituents in aromatic substitution are given wh...
Text Solution
|
- Benzene and naphthalene form an ideal solution at room temperature. Fo...
Text Solution
|
- In the reaction, HC-=CH+2AgNO(3) overset(NH(4)OH)to X+2NH(4)NO(3)+...
Text Solution
|
- Isopropyl benzene is oxidised in the presence of air to a compound 'A'...
Text Solution
|
- Predict the product in the following reactions
Text Solution
|