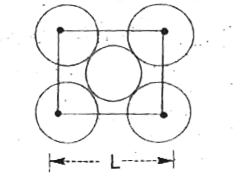
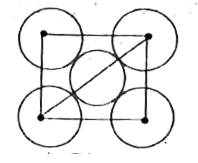
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE) REASONING TYPE QUESTIONS|1 VideosSOLID STATE
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE) COMPREHENSION|4 VideosSOLID STATE
FIITJEE|Exercise SOLVED PROBLEMS (SUBJECTIVE)|17 VideosQUALITATIVE ANALYSIS
FIITJEE|Exercise Single interger answer type|3 VideosSTOICHIOMETRY AND BALANCING REDOX REACTION
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|5 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-SOLID STATE-SOLVED PROBLEMS (OBJECTIVE)
- The packing efficiency of the two - dimensional square unit cell show ...
Text Solution
|
- If 'a' stands for the edge length of the cubic systems: simple cubic,b...
Text Solution
|
- In a compound ,atoms of element Y from ccp lattice and those of elemen...
Text Solution
|
- The density of a certain solid AB (formula mass =119) is 2.75 g//cm^(3...
Text Solution
|
- Which defect cause decrease in the density of crystal?
Text Solution
|
- A solid has a b.c.c. structure . If the distance of closest approach b...
Text Solution
|
- KCl crystallises in the same type of lattice as does NaCl Given that r...
Text Solution
|
- A compound alloy of gold and copper crystallizes in a cubic lattice in...
Text Solution
|
- In which type of viods magnesium and silicon is present respectively i...
Text Solution
|
- The composition of a sample of wustite is Fe(0.93)O(1.0). What is the ...
Text Solution
|
- In a compound AB the ionic radii of A^(+) and B^(-) are 88 pm and 200 ...
Text Solution
|
- In a solid AB having the NaCl structure, A atom occupies the corners o...
Text Solution
|
- A compound formed by elements A and B crystallizes in cubic structure,...
Text Solution
|
- Potassium has a bcc structure with nearest neighbour distance 4.52over...
Text Solution
|
- A compound formed by elements A and B has a cubic structure in which A...
Text Solution
|
- CsBr has BCC structure. The length of its side is 4.3 Å. The minimum d...
Text Solution
|
- The edge length of a face centred unit cubic cell is 508 pm. If the ra...
Text Solution
|
- The no. of atoms in 100g of a fcc crystal with density =10.0 g/cc ...
Text Solution
|
- The unit cell of aluminium is a cube with an edge length of 405 pm. Th...
Text Solution
|
- The face centred cell of nickel has an edge length 352.39 pm. The dens...
Text Solution
|