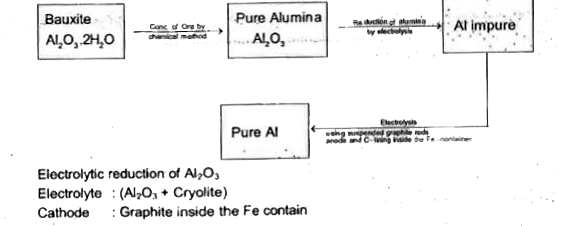
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORES AND METALLURGY
FIITJEE|Exercise MATCHING TYPE SINGLE CORRECT QUESTION|6 VideosORES AND METALLURGY
FIITJEE|Exercise MATRIX-MATCH THE FOLLOWING QUESTION|1 VideosORES AND METALLURGY
FIITJEE|Exercise REASONING TYPE QUESTIONS|13 VideosNUCLEIC ACID AND VITAMIN
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE)|15 VideosPERIODIC PROTERTIES OF ELEMENTS
FIITJEE|Exercise COMPREHENSION|13 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-ORES AND METALLURGY-COMPREHENSION
- Extraction of aluminum can be understood by The purpose of ad...
Text Solution
|
- Extraction of aluminum can be understood by Coke powder is spr...
Text Solution
|
- Extraction of aluminum can be understood by The function of fl...
Text Solution
|
- Extraction of aluminum can be understood by The molten electr...
Text Solution
|
- Extraction of aluminum can be understood by What is wrong if a...
Text Solution
|
- Extraction of aluminum can be understood by Extraction of met...
Text Solution
|
- Extraction of aluminum can be understood by Among the followi...
Text Solution
|
- Extraction of aluminum can be understood by Which of the follo...
Text Solution
|
- Extraction of aluminum can be understood by In Blast Furnace F...
Text Solution
|