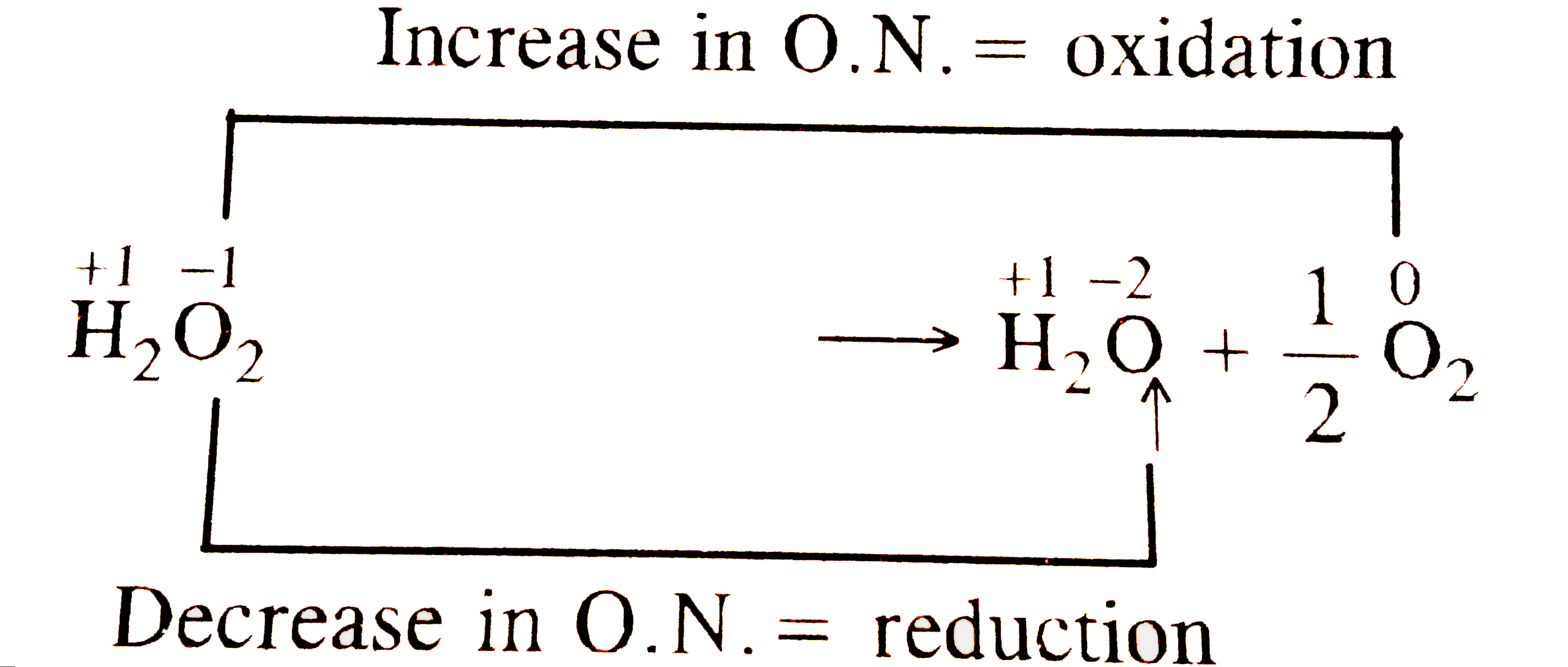Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Hydrogen peroxide can act both as an oxidising as well as reducing age...
Text Solution
|
- Nitric acid acts as an oxidising agent while nitrous acid can act both...
Text Solution
|
- Why hydrogen peroxide acts as an oxidising agent as well as a reducing...
Text Solution
|
- Hydrogen peroxide can act both as an oxidising as well as reducing age...
Text Solution
|
- Which can act as an oxidising as well as a reducing agent ?
Text Solution
|
- Hydrogen peroxide can function as an oxidising agent as well as reduci...
Text Solution
|
- अपनी अभिक्रियाओं में सल्फर डाइऑक्साइड तथा हाइड्रोजन परॉक्साइड ऑक्सीकार...
Text Solution
|
- NO acts both as an oxidising as well as a reducing agent - why ?
Text Solution
|
- दो अथवा तीन वाक्यों में निम्न का कारण स्पष्ट कीजिए | "हाइड्रोजन परॉक...
Text Solution
|