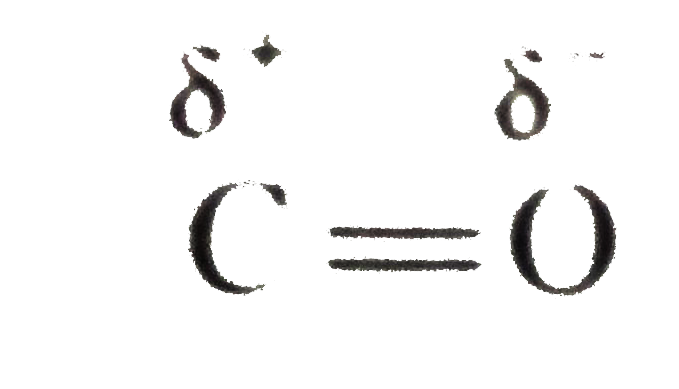Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES AND KETONES
OP TANDON|Exercise ILLUSTRATIONS OF OBJECTIVE QUESTIONS|12 VideosALDEHYDES AND KETONES
OP TANDON|Exercise PROBLEMS FOR PRACTICE|14 VideosALCOHOLS, PHENOLS AND ETHERS
OP TANDON|Exercise SINGLE INETER ANSWER TYPE QUESTIONS / ALCOHOLS AND PHENOLS|15 VideosBASIC PRINCIPLES
OP TANDON|Exercise SECTION V INTEGER ANSWER TYPE QUESTION|3 Videos
OP TANDON-ALDEHYDES AND KETONES -SINGLE INTEGER ANSWER TYPE QUESTIONS
- Explain why aldehydes are more reactios than ketones.
Text Solution
|
- Isomeric aldehydes and ketones having the formula C(5)H(10)O are :
Text Solution
|
- How many of the osomeric ketones having the molecular formula C(6)H(12...
Text Solution
|
- if ethanedial (HOC-COH) is teated with excess of HCN (aq) followed by...
Text Solution
|
- H(2)C=O+D(2)C=O+cons. NaOHrarr"Cannizzaro's reaction." How many diff...
Text Solution
|
- Find out the number of products obtained by crossed Cannizzaro's react...
Text Solution
|
- Of the following carbonyl compounds, how many would give aldol condens...
Text Solution
|
- In the reaction given below, how many different oximes would be fomred...
Text Solution
|
- In the scheme given below. The total number of intramolecular aldol co...
Text Solution
|
- Of the following carbonyl compounds, many would give Cannizzaro reacti...
Text Solution
|
- R-overset(O)overset(||)C-Roverset(?)rarr-R Idenitify the number of r...
Text Solution
|