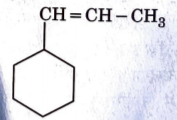
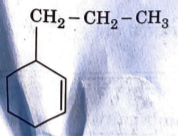
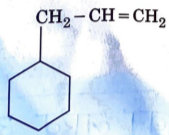
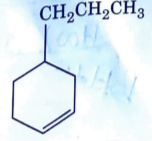
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-NEET 2020-All Questions
- Which of the following amine will give the carbylamine test ?
Text Solution
|
- An alkene on ozonolysis gives methanal as one of the product. Its stru...
Text Solution
|
- Match the following and identify the correct option.
Text Solution
|
- The freezing point depression constant of benzene is 5.12 K kg mol^(-1...
Text Solution
|
- During the electrolysis of a dilute solution of sulphuric acid, what s...
Text Solution
|
- Identify compound X in the following sequence reactions:
Text Solution
|
- Which one of the following has maximum number of atoms?
Text Solution
|
- Identify the correct statement from the following:
Text Solution
|
- A tertiary butyl carbocation is more stable than a secondary butyl car...
Text Solution
|
- Urea reacts with water to form A which will decompose to form B. B whe...
Text Solution
|
- A mixture of N2 and Ar gases in a cylinder contains 7g of N2 & 8 g of ...
Text Solution
|
- An element has a body centered cubic (bcc) structure with a cell edge ...
Text Solution
|
- The rate constant for a first order reaction is 4.606 xx 10^(-3) s^(-1...
Text Solution
|
- Reaction between acetone and methyl magnesium chloride followed by hyd...
Text Solution
|
- Which of the following set of molecules will have zero diplole moment?
Text Solution
|
- What is the change in oxidation number of carbon in the following reac...
Text Solution
|
- Match the following : Which of the following is correct option ?
Text Solution
|
- Which of the following is not correct about carbon monoxide?
Text Solution
|
- Measuring Zeta potential is useful in determining which property of co...
Text Solution
|
- Which of the following is the correct order of increasing field streng...
Text Solution
|