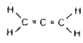A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE|Exercise Assignment(SECTION-A)|60 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE|Exercise Assignment(SECTION B)|3 VideosORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES
AAKASH INSTITUTE|Exercise Try Yourself|23 VideosMOCK_TEST_20
AAKASH INSTITUTE|Exercise EXAMPLE|28 VideosORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUES
AAKASH INSTITUTE|Exercise TRY YOURSELF|28 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-ORGANIC CHEMISTRY : SOME BASIC PRINCIPLES AND TECHNIQUES -Exercise
- The -hybridisations of central and terminal carbon atoms in are respec...
Text Solution
|
- Correct IUPAC name for H(3)C - underset(C(2)H(5))underset(|)(CH) - un...
Text Solution
|
- Which of the following compound has linear shape?
Text Solution
|
- IUPAC name of the compound
Text Solution
|
- IUPAC name of the compound
Text Solution
|
- The molecular formula of the compound
Text Solution
|
- In which of the following compound has only one type of hybridised car...
Text Solution
|
- Which of the following compound has maximum number of primary H-atoms?
Text Solution
|
- Tetravalency of carbon is possible in the case of
Text Solution
|
- The IUPAC name of CH(2) = CH - CH(2) - OH is
Text Solution
|
- Which of the following alkane cannot show isomerism?
Text Solution
|
- The number of structural isomers possible for C(4)H(8) is
Text Solution
|
- IUPAC name of the compound
Text Solution
|
- Which of the following is a heterocyclic compound?
Text Solution
|
- Which of the following is incorrect match?
Text Solution
|
- Which of the following will not show tautomerism?
Text Solution
|
- Which of the following is not the isomer of CH(3)COOH?
Text Solution
|
- Which of the following has maximum -I effect?
Text Solution
|
- Carbon-carbon bond-order in benzene is -1.5 due to
Text Solution
|
- Which of the following is the most acidic?
Text Solution
|