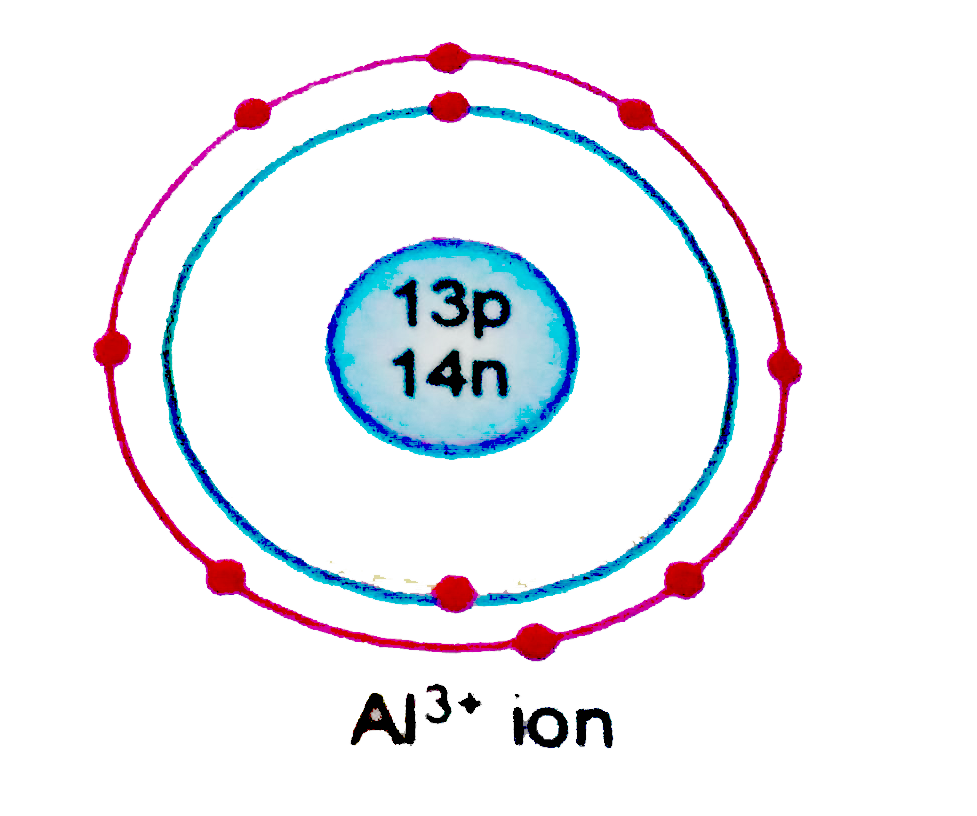
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-STRUCTURE OF ATOM -Long Answer Questions
- Atomic number of Alis 13 and mass number is 27 .Calculate the number o...
Text Solution
|
- Define the terms atomic number and mass number how are they related to...
Text Solution
|
- Describe Rutherford atom model. What are the drawbacks of this model?
Text Solution
|
- Complete the following table:
Text Solution
|
- What are isotopes? What is common in the isotopes of an element . Give...
Text Solution
|
- How will you account for the following ? (i) an atom is electrically...
Text Solution
|
- An ion M^(3+) contains 10 electrons and 14 neutrons what are the aomic...
Text Solution
|