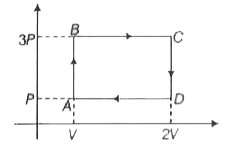
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-AP EAMCET ENGINEERING ENTRANCE EXAM QUESTION PAPER 2017 (SOLVED)-Physics
- A monoatomic ideal gas through a cyclic process as shown in the ...
Text Solution
|
- Two situations are shown in fig. (a) and (b) In each case m(1) =...
Text Solution
|
- Three uniform thin aluminum rods each of length 2 m form an equilatera...
Text Solution
|
- An infinitely long thin straight wire has uniform linear charge densit...
Text Solution
|
- An electrostatic paint sprayer has a metal sphere of diameter 18 cm an...
Text Solution
|
- A body is projected from the top of a tower with a velocity bar(u) ...
Text Solution
|
- Two long parallel conducting wires carrying currents are seperated by ...
Text Solution
|
- A Zener diode voltage regulator operated in the range 120 V - 180 V ...
Text Solution
|
- Equation of a projectile is given by y = Px - Qx^(2) , where P and...
Text Solution
|
- The transverse displacement of a string of a linear dinsity 0 . 0 1kgm...
Text Solution
|
- A girl of mass 50 kg swinging on a cardle. If she moves with a veloci...
Text Solution
|
- Two point surces S(1) and S(2) are 24 cm apart. Where should a convex...
Text Solution
|
- Assertion (A) It is more difficult to push a magnet into a coil with ...
Text Solution
|
- A wall is made of equally thick layers P and Q of different materials...
Text Solution
|
- In the determination of the internal resistance of a cell with a pot...
Text Solution
|
- The half life of a stream of radioactive particles moving along as st...
Text Solution
|
- The process of recovering the modulating signal from the modulated ca...
Text Solution
|
- Two bodies of masses 4m and 9m are seperated by distance 'r' . The gra...
Text Solution
|
- If the charge on the capacitor is 1 mC in the given circuit, the...
Text Solution
|
- The amplitude of electric field in an electromagnetic wave is 60 Vm^(...
Text Solution
|