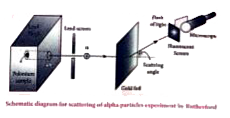
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Explain the results of Rutherford alpha-particle scattering experiment...
Text Solution
|
- From the alpha - particle scattering experiment Rutherford concluded t...
Text Solution
|
- From the alpha-particle scattering experiment, Rutherford conculded th...
Text Solution
|
- Rutherford's alpha particle scattering experiment was responsible for ...
Text Solution
|
- रदरफोर्ड के alpha- कणों के प्रकीर्णन -प्रयोग में, alpha-कणों का स्त्र...
Text Solution
|
- अल्फा कणों के रदरफोर्ड प्रकीर्णन प्रयोग से निष्कर्ष निकलता है कि :
Text Solution
|
- Explain the results of Rutherford alpha-particle scattering experiment...
Text Solution
|
- Explain the results of Rutherford alpha -particle scattering experimen...
Text Solution
|
- Explain the results of Rutherford alpha-particle scattering experiment...
Text Solution
|