Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
NCERT TELUGU|Exercise Intext Question Intext Question|9 VideosHALOALKANES AND HALOARENES
NCERT TELUGU|Exercise Exercises|21 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
NCERT TELUGU|Exercise Exercises|27 VideosORGANIC NITROGEN COMPOUNDS
NCERT TELUGU|Exercise Diazonium chloride|4 Videos
Similar Questions
Explore conceptually related problems
NCERT TELUGU-HALOALKANES AND HALOARENES-Exercises
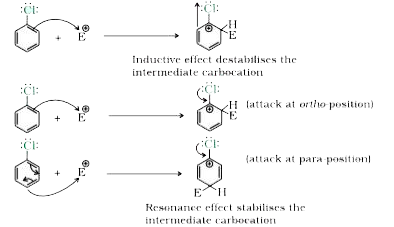
- Although chlorine is an electron withdrawing group , yet it is ortho-,...
Text Solution
|
- Give the IUPAC names of the following compounds: (i) CH(3)CH(Cl)CH(B...
Text Solution
|
- Write the structures of the following organic halides . 1-Bromo-4 se...
Text Solution
|
- Which one of the following has highest dipole moment ? (i) CH(2) Cl...
Text Solution
|
- A hydrocarbon C(5)H(10) does not react with chlorine in dark but gives...
Text Solution
|
- Write the isomers of the compound having molecular formula C(4) H(9) B...
Text Solution
|
- Write the equations for the preparation of 1-iodobutane from (i) 1-b...
Text Solution
|
- What are ambident nucleophiles ?
Text Solution
|
- Which compound in each of the following pairs will react faster in S(N...
Text Solution
|
- Predict all the alkenes that would be formed by dehydrohalogenation of...
Text Solution
|
- Explain how the conversions are carried out : Ethanol to But-1-yne
Text Solution
|
- Explain why the dipole moment of chlorobenzene is lower than that of c...
Text Solution
|
- Give the uses of freon 12, DDT, carbon tetrachloride and iodoform.
Text Solution
|
- Write the structure of the major organic product in each of the follow...
Text Solution
|
- Write the mechanism of the following reaction: nBuBr +KCNoverset(EtO...
Text Solution
|
- Arrange the compounds of each set in order of reactivity towards S(N)2...
Text Solution
|
- Out of C(6)H(5) CH(2) Cl and C(6) H(5) CH Cl C(6) H(5) , which is more...
Text Solution
|
- p-Dichlorobenzene has higher m.p. and solubility than those of o- and ...
Text Solution
|
- Explain how the conversions are carried out : Ethanol to But-1-yne
Text Solution
|
- Treatment of alkyl halides with aq. KOH leads to the formation of alco...
Text Solution
|
- Primary alkyl halide C(4)H(9)Br (a) reacted with alcoholic KOH to give...
Text Solution
|