Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(3 MARK QUESTIONS)|39 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(5 MARK QUESTIONS)|13 VideosPERIODIC CLASSIFICATION OF ELEMENTS
FULL MARKS|Exercise Additional Questions(IX Choose the correct statement)|1 VideosHYDROGEN
FULL MARKS|Exercise Additional Questions Solved 5-Mark Questions|10 VideosPHYSICAL AND CHEMICAL EQUILIBRIUM
FULL MARKS|Exercise ADDITIONAL QUESTIONS SOLVED (NUMERICAL PROBLEMS)|3 Videos
Similar Questions
Explore conceptually related problems
FULL MARKS-PERIODIC CLASSIFICATION OF ELEMENTS -Additional Questions(2 MARK QUESTIONS)
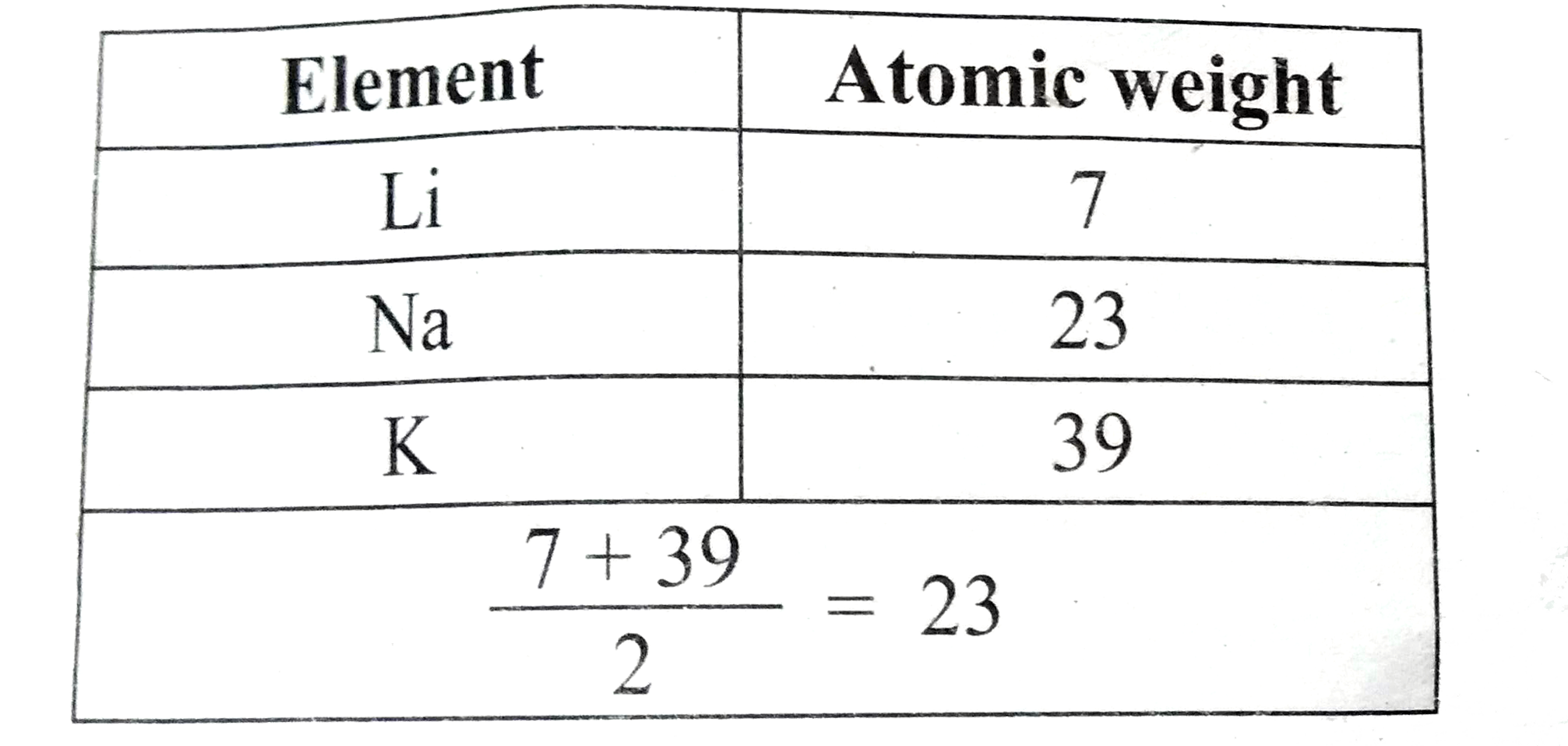
- State Johann Dobereiner's law of triads.
Text Solution
|
- Write a note about chancourtois classification .
Text Solution
|
- State the Newland's law of octaves.
Text Solution
|
- State Mendeleev's period law.
Text Solution
|
- Explain about the relationship between the atomic number of an element...
Text Solution
|
- State modern periodic law.
Text Solution
|
- What are the anomalies of the long form of periodic table?
Text Solution
|
- Mention the names of the elements with atomic number 101 ,102,109 and ...
Text Solution
|
- Write a note about the electronic configuration of elements in groups.
Text Solution
|
- Give the name and electronic configuration of elements of 1^(st) group...
Text Solution
|
- Write any two characteristic properties of alkali metals.
Text Solution
|
- Write any two characteristic properties of alkaline earth metals.
Text Solution
|
- Groups from 13 to 18 in the periodic table are called p-block elements...
Text Solution
|
- Why noble gases do not show much of chemical reactivity?
Text Solution
|
- Halogens and chalcogens have highly negative electron gain enthalpies....
Text Solution
|
- What are d-block elements? Why are they called so?
Text Solution
|
- Elements Zn, Cd and Hg with electronic configuration (n-1)d^(10) ns^(2...
Text Solution
|
- Why Zn, Cd and Hg are considered as soft metals?
Text Solution
|
- Why d- block elements are known as transition element ?
Text Solution
|
- What are f-block elements? How many series are there? Why they are cal...
Text Solution
|