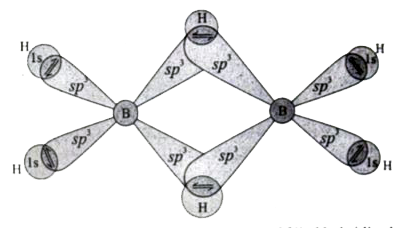
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
FULL MARKS-SAMPLE PAPER - 7 (SOLVED) -PART-IV
- (i) Explain the role of carbon monoxide in the purification of nickel?...
Text Solution
|
- (i) What type of hybridisation occur in 1. BrF(5) 2. BrF(3) (ii) Mos...
Text Solution
|
- (i) Why tetrahedral complexes do not exhibit geometrical isomerism. ...
Text Solution
|
- Explain AAAA and ABABA and ABCABC type of three dimensional packing wi...
Text Solution
|
- (i) The rate constant for a first order reaction is 1.54 times 10^(-3)...
Text Solution
|
- (i) What is buffer solution? Give an example for an acidic buffer and ...
Text Solution
|
- Describe about lead storage battery construction and its uses.
Text Solution
|
- (i) What happens when m-cresol is treated with acidic solution of sodi...
Text Solution
|
- (i) An aromatic compound 'A' on treatment with aqueous ammonia and hea...
Text Solution
|
- (i) Explain the mechanism of enzyme action? (ii) Which chemical is r...
Text Solution
|