Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
XII BOARDS PREVIOUS YEAR-XII BOARDS-Set -II
- What type of stoichiometric defect is shown by AgCl ?
Text Solution
|
- Write the IUPAC name of CH3CH=CH-underset(Br)underset(|)overset(CH3)o...
Text Solution
|
- What type of bonding helps in stabilising the alpha-helix structure of...
Text Solution
|
- What inspired N. Bartlett for carrying out reaction between Xe and PtF...
Text Solution
|
- What happens when ethyl chloride is treated with aqueous KOH?
Text Solution
|
- Write the structure of 4-chloropentan-2-one
Text Solution
|
- How will you convert the following? (i) Propan - 2 - ol to propanone....
Text Solution
|
- What is the difference between oil/water (O/W) type and water/oil (W/O...
Text Solution
|
- (a) Which of the following ores can be concentrated by froth floatatio...
Text Solution
|
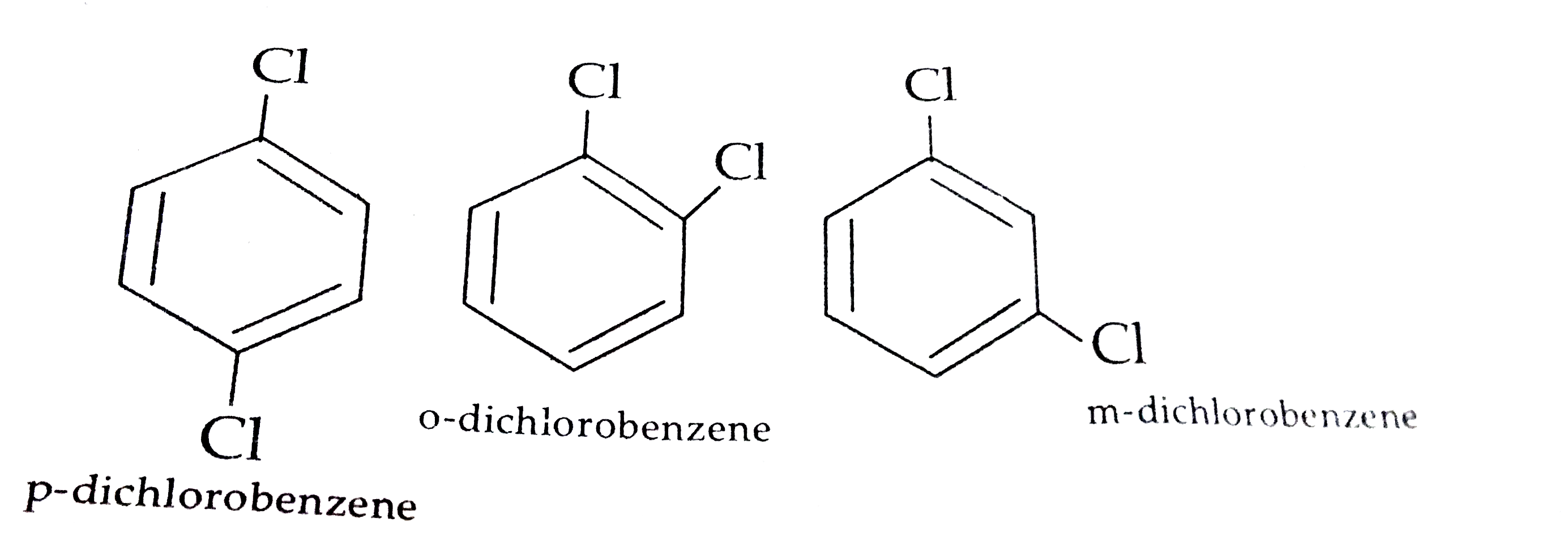
- (a) Why does p-dichlorobenzene have a higher m.p than its o-and m-isom...
Text Solution
|
- Write the names and structures of the monomers of the following polyme...
Text Solution
|