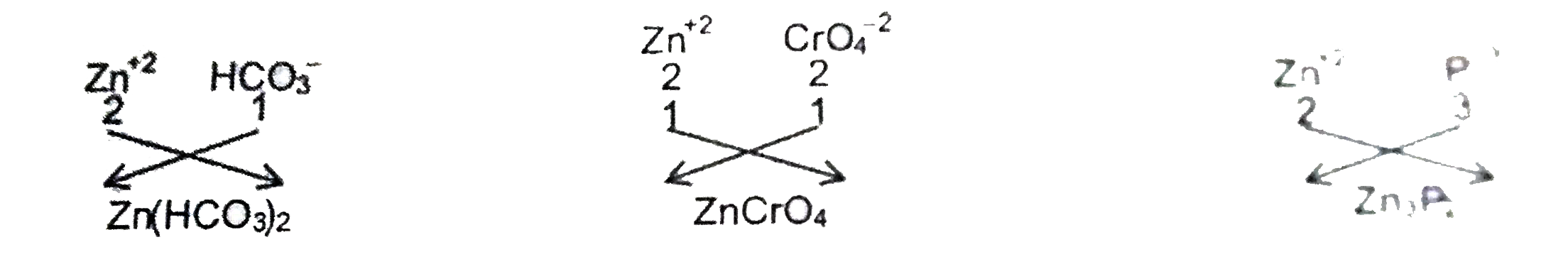
Text Solution
Verified by Experts
Topper's Solved these Questions
ATOMIC STRUCTURE AND TRANSFORMATION OF MATTER
PEARSON IIT JEE FOUNDATION|Exercise Assessment Test 1|15 VideosATOMIC STRUCTURE AND TRANSFORMATION OF MATTER
PEARSON IIT JEE FOUNDATION|Exercise Assessment Test 2|15 VideosATOMIC STRUCTURE AND TRANSFORMATION OF MATTER
PEARSON IIT JEE FOUNDATION|Exercise Level -2|35 VideosAIR AND OXYGEN
PEARSON IIT JEE FOUNDATION|Exercise Assemssment Test-2|15 VideosCHEMISTRY IN DAILY LIFE
PEARSON IIT JEE FOUNDATION|Exercise MOCK TEST|25 Videos
Similar Questions
Explore conceptually related problems
PEARSON IIT JEE FOUNDATION-ATOMIC STRUCTURE AND TRANSFORMATION OF MATTER-Level -3
- An atom of an element 'X' has three completely filled shell . The ra...
Text Solution
|
- A metal (M) and a non - metal (X) form a compound with formula M(4)X...
Text Solution
|
- Two metals A and B possess the same number of electrons in L shell an...
Text Solution
|
- Number of atoms on either side of a chemical equation is balanced by k...
Text Solution
|
- An atom of an element has two shells with electrons.The valence shell ...
Text Solution
|