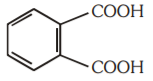
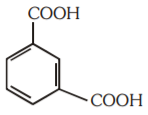
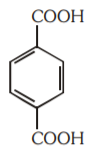
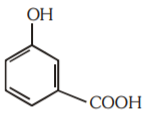
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-RE-NEET 2020-All Questions
- Identify the reaction from having top position in EMF series according...
Text Solution
|
- Match the element in column I with methods of purification in columb I...
Text Solution
|
- Which among the following salt solutions is basic in nature?
Text Solution
|
- In which of the sols, the colloidal particles are with negative charge...
Text Solution
|
- Which of the following acid will form an (a) Anhydride on heating and ...
Text Solution
|
- In a typical fuel cell, the reactants(R) and product (P) are:
Text Solution
|
- In collision theory of chemical reaction, Z(AB) represents
Text Solution
|
- Which of the following statement is not true abount glucose?
Text Solution
|
- The potential energy(y) curve for H2 formation as a function of inrtnu...
Text Solution
|
- Identify compound (A) in the following reaction:
Text Solution
|
- How many sp^2 hybridised carbon atoms and pi bonds respectively are pr...
Text Solution
|
- At standard conditions, if the change in the enthalpy for the followin...
Text Solution
|
- The minimum pressure required to compress 600dm^3 of a gas at 1bar to ...
Text Solution
|
- What is the role of gypsum, CaSo4.2H2O in setting of cement? Identify ...
Text Solution
|
- Which of the following oxide is amphoteric in nature?
Text Solution
|
- Which one of the following reactions does not come under hydrolysis ty...
Text Solution
|
- Which one of the following compounds shows both, Frenkel as well as Sc...
Text Solution
|
- One mole of carbon atom weighs 12g, the number of atoms in it is equal...
Text Solution
|
- Isotonic solutions have same
Text Solution
|
- The solubility product for a salt of the type AB is 4xx10^(-8). What i...
Text Solution
|