Specific heat capacity
Specific heat capacity
Text Solution
Verified by Experts
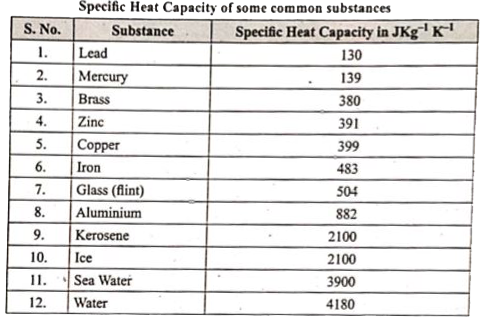
Specific heat capacity of a substance is defined as the amount of heat required to raise the temperature of 1 kg of the substance by `1^@C` or 1 K. The SI unit of specific heat capacity is `JKg^(-1) K^(-1)` .

Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
Figure shows a large tank of water at a constant temperature theta_(0) and a small vessel containing a mass m of water at an initial temperature theta_1 (lt theta_(0)) . A metal rod of length L , area of cross section A and thermal conductivity K connects the two vessels. Find the time taken for the temperature of the water in the smaller vessel to become theta_(2) (theta_(1)lt theta_(2)lt theta_(0)) . Specific heat capacity of water is s and all other heat capacities are negligible.
Types: A Q= mcDelta (Heat energy during change in temperature) Q = mL (Heat energy during change of state ) Note : Specific heat capacity (c) =1 cal // g^(@)C = 1 kcal // kg^(@) C Delta T = Higher temperature - Lower temperature . How much heat energy is necessary to raise the temperature of 5kg of water form 20^(@) C to 100^(@) C.
An experiment is performed to measure the molar heat capacity of a gas at constant pressure using Regnault's method. The gas is initially contained in a cubical reservoir of size 40 cm xx 40 cm xx 40 cm xx at 600 kPa at 27^0C . A part of the gas is brought out, heated to 100^0C and is passed through a calorimeter at constant pressure. The water equivalent of the calorimeter and its contents increases from 20^0C to 30^0C during the experiment and the pressure in the reservoir decresases to 525 kPa . Specific heat capacity of water = 4200 J kg^(-1) K^(1). Calculate the molar heat capacity Cp from these data.
A hot body placed in a surrounding of temperature theta_(0) obeys Newton's law of cooling (d theta)/(dt)=-k(theta-theta_(0)) . Its temperature at t=0 is theta_(1) the specific heat capacity of the body is s and its mass is m . Find (a) the maximum heat that the body can lose and (b) the time starting from t=0 in which it will lose 90% of this maximum heat.
Calculate the increase in the internal energy of 10 g of water when it is heated from 0^(0)C to 100^(0)C and converted into steam at 100 kPa. The density of steam =0.6 kg m^(-3) specific heat capacity of water =4200 J kg^(-1 ^(0)C^(-3) latent heat of vaporization of water =2.25xx10^(6) J kg^(-1)
In Joly's differential steam calorimeter, 3g of an ideal gas is ccontained in a rigid closed sphere at 20^@C . The sphere is heated by steam at 100^@C and it is found that an extra 0.095 g of steam has condensed into water as the temperature of the gas becomes constant. Calculate the specific heat capacity of the gas in (Jg^(-1) K^(-1). The latent heat of vaporization of water = 540 cal g ^(-1) .
Four 2 cmxx2 cmxx 2cm cubes of ice are taken from a refrigerator and put in 200ml of a drink at 10^(@)C Find the temperature of the drink when thermal equilibrium is attained in it.Density of ice = 900 kg m^(-8) , density of the drink = 1000kgm^(-8) , specific heat capacity of the drink = 4200 J kg^(-1)K^(-1) ,latent heat of fusion of ice = 3.4 xx 10^(6)Jkg^(-1)
A plate of area 10 cm^2 is to be electroplated with copper (density 9000 kg m^(-3) to a thickness of 10 micrometres on both sides, using a cell of 12 V . Calculate the energy spend by the cell in the process of deposition. If this energy is used to heat 100 g of water, calculate the rise in the temperature of the water. ECE of copper = 3 xx 10^(-7) kg C^(-1) and specific heat capacity of water = 4200 J kg^(-1) K^(-1) .
Find the change in the internal energy of 2 kg of water as it heated from 0^(0)C to 4^(0)C . The specific heat capacity of water is 4200 J kg^(-1) K^(-1) and its densities at 0^(0)C and 4^(0)C are 999.9 kg m^(-3) and 1000 kg m^(-3) respectively. atmospheric pressure =10^(5) Pa.
A metal ball of mass 1kg is heated by means of a 20W heater in a room at 20^(@)C . The temperature of the ball becomes steady at 50^(@)C . (a) Find the rate of loss of heat to the surrounding when the ball is at 50^(@)C . (b) Assuming Newton's law of cooling, calculate the rate of loss of heat to the surrounding when the ball is at 30^(@)C . (c) Assume that the temperature of the ball rises uniformly from 20^(@)C to 30^(@)C in 5 minutes. Find the total loss of heat to the surrounding during this period. (d) Calculate the specific heat capacity of the metal.