Text Solution
Verified by Experts
Topper's Solved these Questions
ORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise NCERT FILE (NCERT IN-TEXT QUESTIONS)|9 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise NCERT FILE NCERT (TEXTBOOK EXERCISES)|14 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
MODERN PUBLICATION|Exercise PRACTICE PROBLEMS|3 VideosHALOALKANES AND HALOARENES
MODERN PUBLICATION|Exercise UNIT PRACTICE TEST|11 VideosPOLYMERS
MODERN PUBLICATION|Exercise Competition file (OBJECTIVE TYPE QUESTIONS) (C. MULTIPLE CHOICE QUESTIONS)(Integer Type Questions)|6 Videos
Similar Questions
Explore conceptually related problems
MODERN PUBLICATION-ORGANIC COMPOUNDS CONTAINING NITROGEN -CONCEPTUAL QUESTIONS
- Although boron trifluoride adds on trimethylamine but it does not add ...
Text Solution
|
- Why does silver chloride dissolve in methylamine solution ?
Text Solution
|
- Why does the reactivity of NH(2) get reduced in acetanilide ?
Text Solution
|
- Although trimethyl amine and n-propylamine have the same molecular mas...
Text Solution
|
- Sulphanilic acid is soluble in dil. NaOH but not in dil. HCl. Explain.
Text Solution
|
- Glycine exists as NH(3)""^(+)CH(2)COO^(-) , zwitter ion but anthranili...
Text Solution
|
- Tertiary amines do not undergo acylation. Explain.
Text Solution
|
- Arrange the following sets in order of their basic strength in aqueous...
Text Solution
|
- (i) Name the main product when aniline is heated with alcoholic KOH an...
Text Solution
|
- Why do amines act as nucleophiles?
Text Solution
|
- Aniline does not undergo Friedel Crafts alkylation. Explain.
Text Solution
|
- Although —NH(2) group is an ortho and para directing group, nitration ...
Text Solution
|
- The presence of a base is needed in the ammonolysis of alkyl halides. ...
Text Solution
|
- Why cannot be aromatic primary amines prepared by Gabriel pthalimide s...
Text Solution
|
- Suggest a structural formula of a compound having molecular formula C(...
Text Solution
|
- Why does methylamine has lower boiling point than methanol ?
Text Solution
|
- Identify A, B, C and D in the following conversions: A underset(0-5^...
Text Solution
|
- Arrange the following compounds in the decreasing order of basicity :
Text Solution
|
- Name the following reactions: (i) C(6)H(5)N(2)""^(+)Cl^(-) overset(C...
Text Solution
|
- Arrange the following in the increasing order of their pK(b) values. ...
Text Solution
|
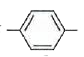 `SO_(3)^(-)`. In the presence of dil. NaOH, the weakly acidic `NH_(3)^(+)` group transfers its `H^(+)` to `OH^(-)` to form a soluble p- `NH_(2)C_(6)H_(4)SO_(3)^(-)Na^(+)`. On the other hand, `SO_(3)^(-)` group is very weak base and therefore, does not accept a proton from dil. HCl to form p- `NH_(3)^(+)C_(6)H_(4)SO_(3)H`. Hence, it does not dissolve in dil. HCI.
`SO_(3)^(-)`. In the presence of dil. NaOH, the weakly acidic `NH_(3)^(+)` group transfers its `H^(+)` to `OH^(-)` to form a soluble p- `NH_(2)C_(6)H_(4)SO_(3)^(-)Na^(+)`. On the other hand, `SO_(3)^(-)` group is very weak base and therefore, does not accept a proton from dil. HCl to form p- `NH_(3)^(+)C_(6)H_(4)SO_(3)H`. Hence, it does not dissolve in dil. HCI.