Text Solution
Verified by Experts
Topper's Solved these Questions
ACIDS, BASES AND SALTS
VK GLOBAL PUBLICATION|Exercise HOTS (Higher Order Thinking Skills )|5 VideosACIDS, BASES AND SALTS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (Very Short Answer Questions (1 mark))|6 VideosACIDS, BASES AND SALTS
VK GLOBAL PUBLICATION|Exercise SHORT ANSWER QUESTIONS - II (3 marks)|13 VideosCARBON AND ITS COMPOUNDS
VK GLOBAL PUBLICATION|Exercise PROFICIENCY EXERCISE (Long Answer Questions)|6 Videos
Similar Questions
Explore conceptually related problems
VK GLOBAL PUBLICATION-ACIDS, BASES AND SALTS -LONG ANSWER QUESTIONS (5 marks)
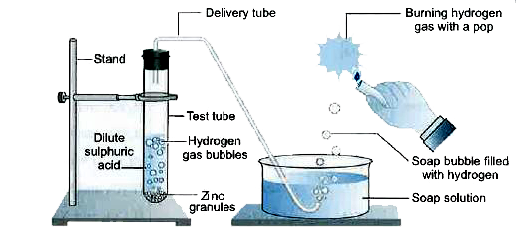
- (i) In the following schematic diagram for the preparation of hydrogen...
Text Solution
|
- A metal carbonate X on reacting with an acid gives a gas which when p...
Text Solution
|
- Write the formulae of the salts given below : Potassium sulphate , s...
Text Solution
|
- A sulphate salt of group 2 element of the periodic tables is a white s...
Text Solution
|