Text Solution
Verified by Experts
|
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Solved Problems|37 VideosView PlaylistAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Exercises Subjective Type|4 VideosView PlaylistAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Archives Subjective|18 VideosView PlaylistAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY|Exercise Short Answer Type|179 VideosView PlaylistBIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Analytical And Descriptive)|8 VideosView Playlist
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY-AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES -Solved Examples
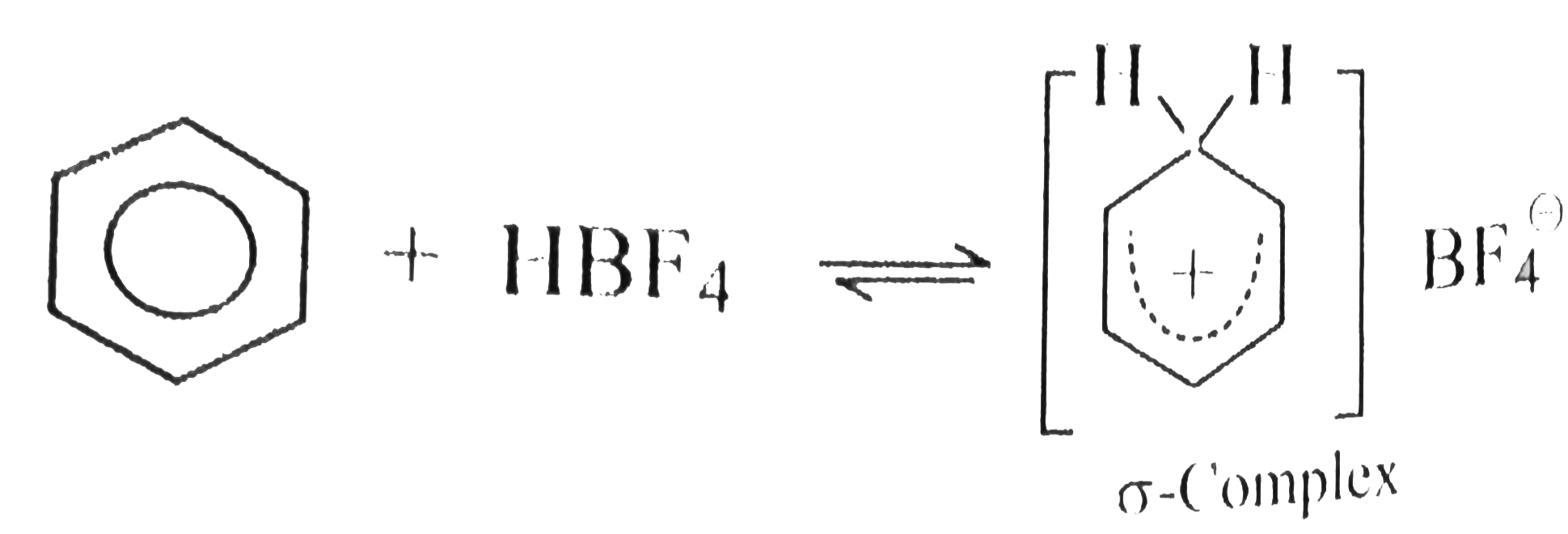
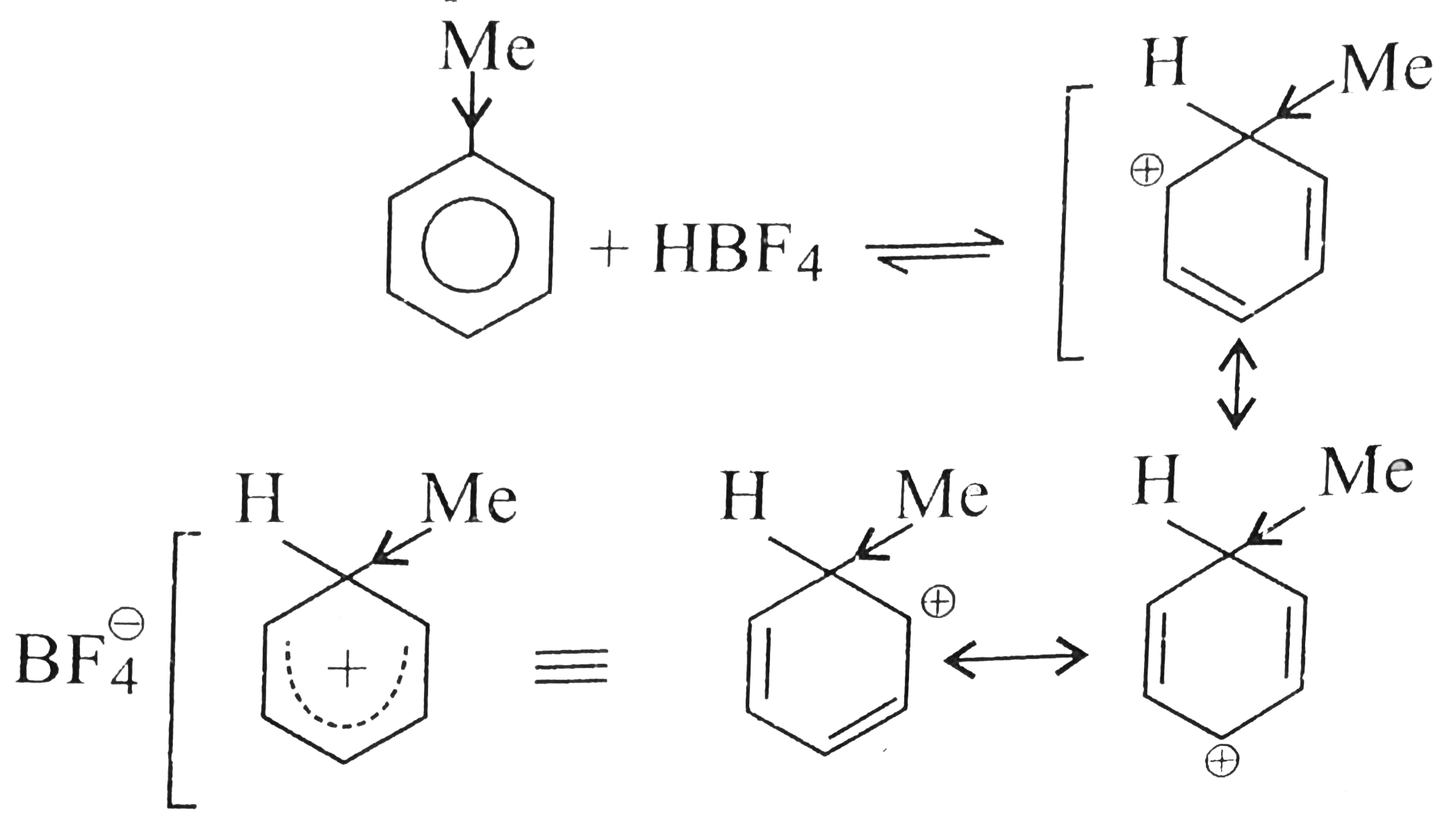
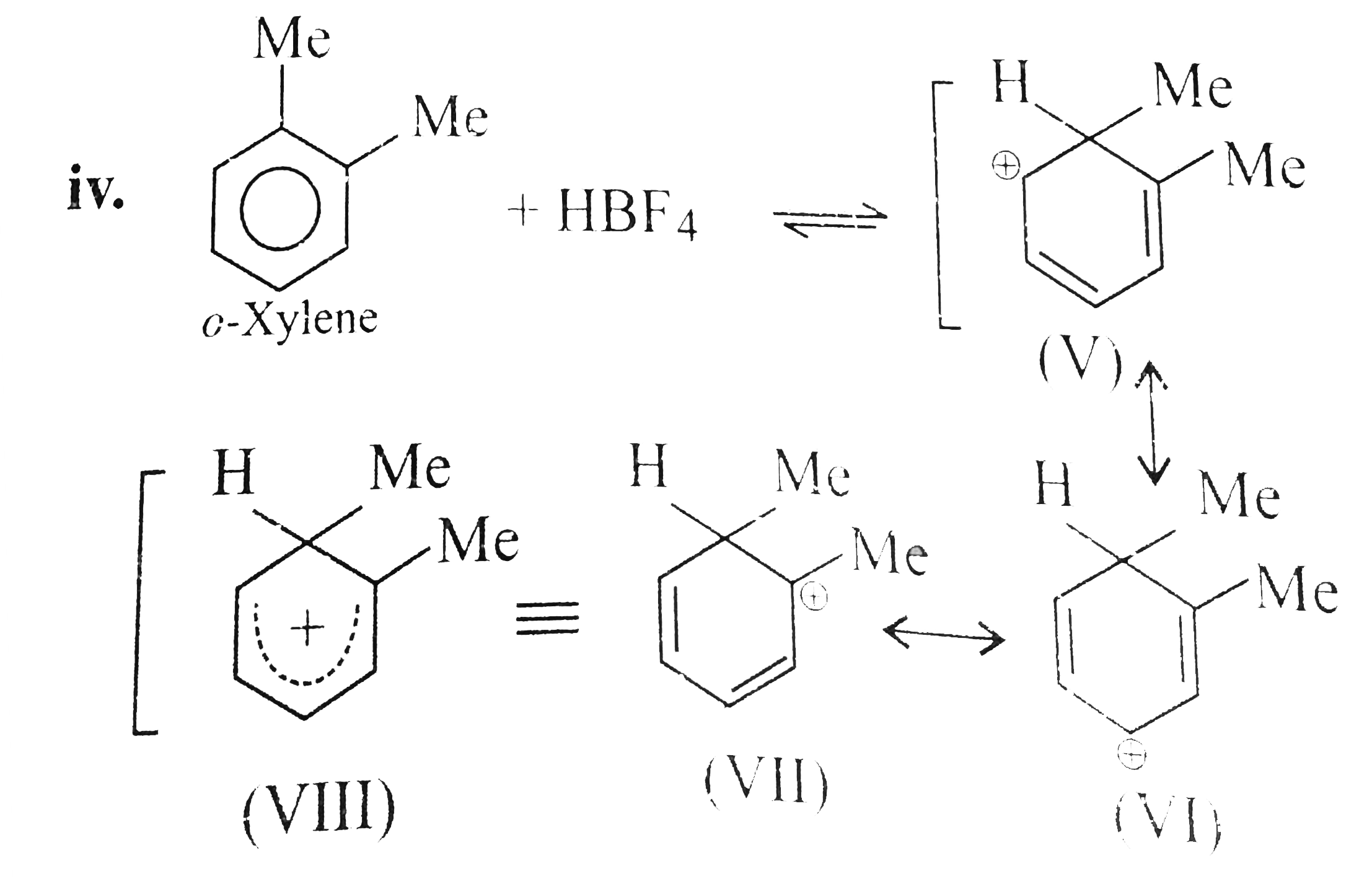
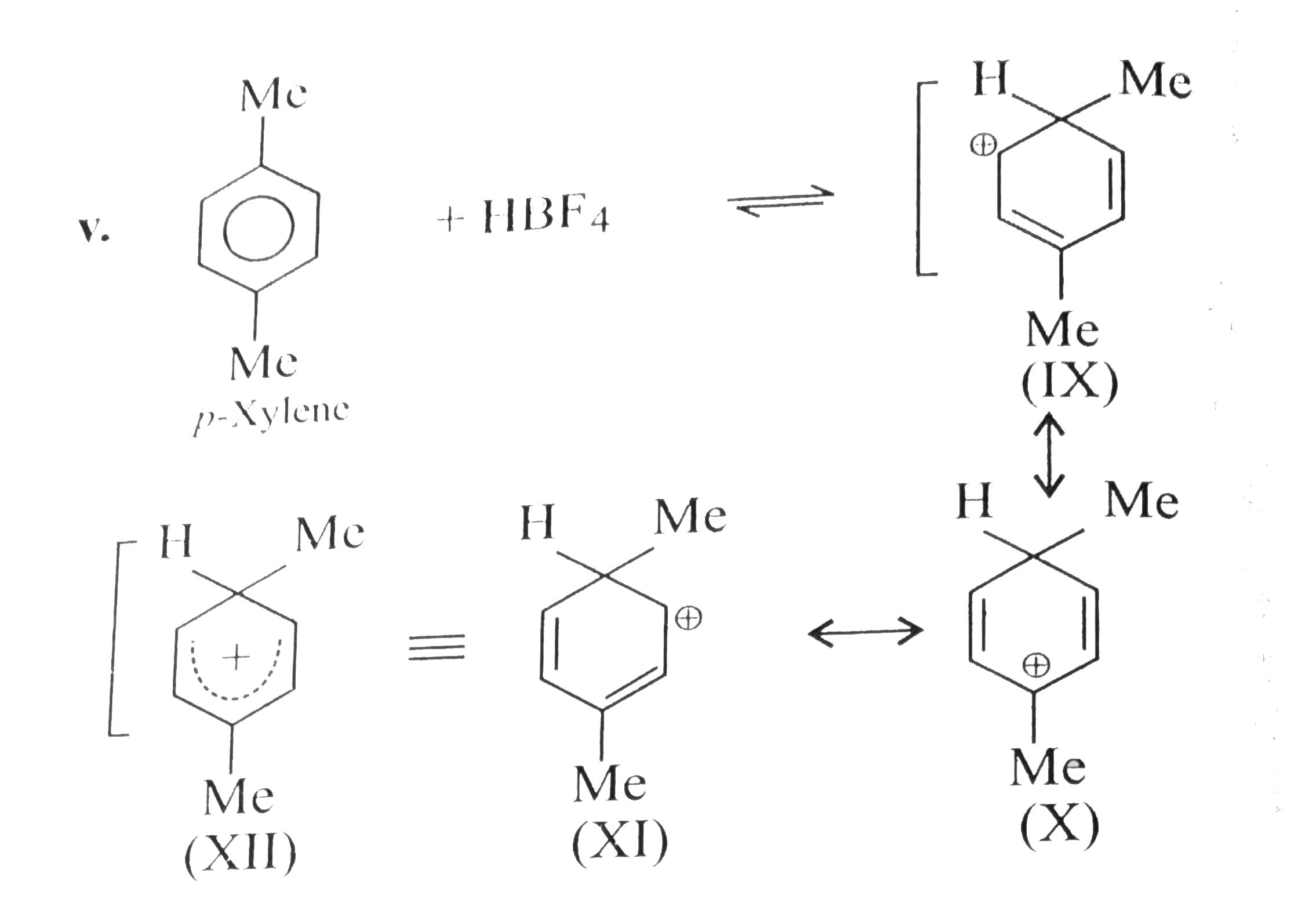
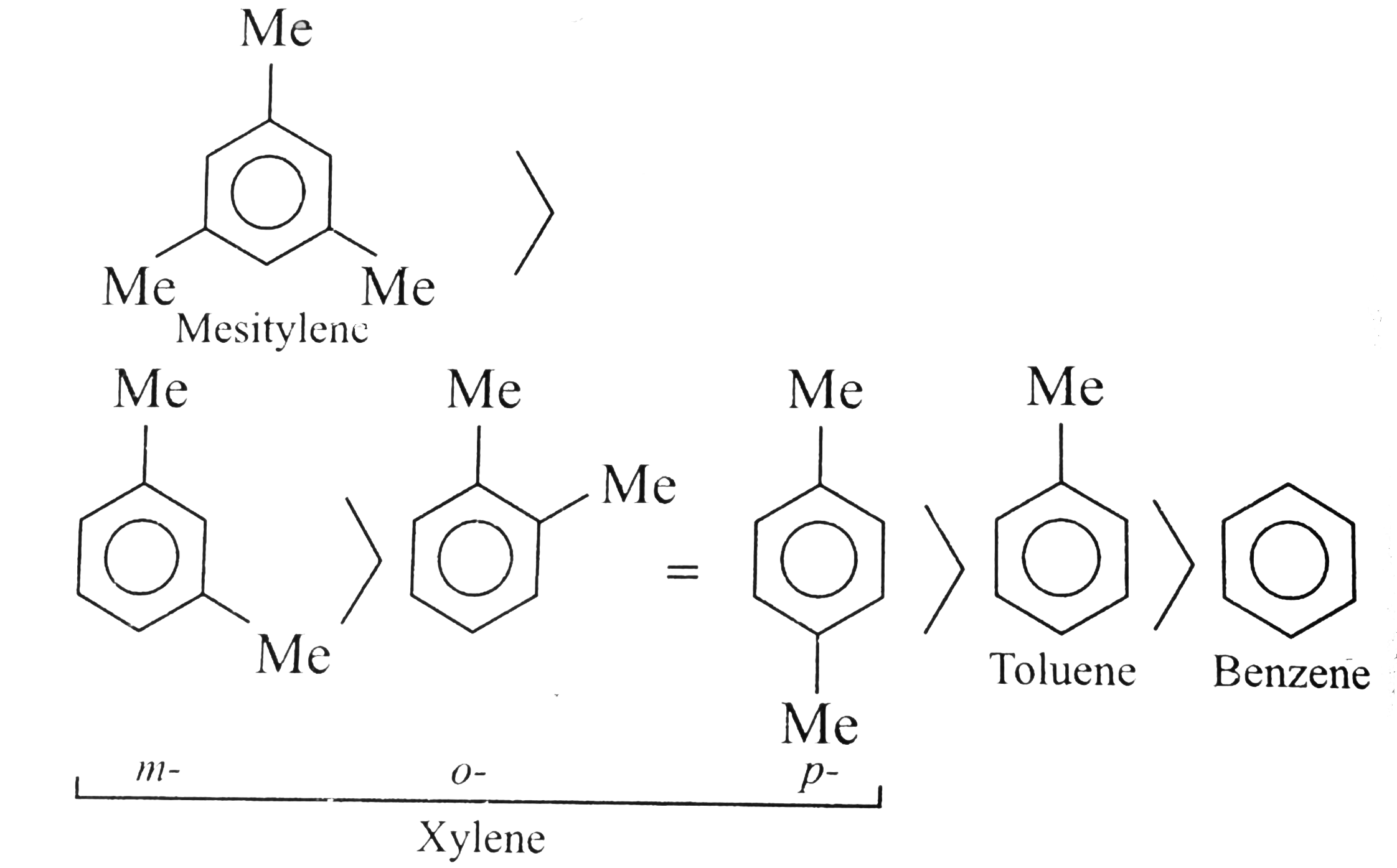
- Benzene, touene, xylene (o,m,p) and mesitylene dissolve in HBF(4) to f...
Text Solution
|
Playing Now - Complete the following :
Text Solution
|
Play - a. The treatment of RX with aqueous KOH leads to the information of al...
Text Solution
|
Play - Give the decreasing order of following with their properties as indica...
Text Solution
|
Play - Distingulish between the following compounds: a. I. m- Iosotoluene ...
Text Solution
|
Play - Complete the following reactions:
Text Solution
|
Play - Indicate the position where ArSN reaction will take place and explain ...
06:00
|
Play - Compound (B) is an ismoer of (A0. Compound (B) shows positive iodofo...
04:01
|
Play Text Solution
|
Play- a. Account for the rapid rate of ehanoulysis id , although it is 1^(o...
Text Solution
|
Play - I. Give the product of debromnination of with Kl in acetone solution ...
Text Solution
|
Play - Syntesise the following: a. Cyclohexanol (A) to 1,2,3- tridenuterocy...
Text Solution
|
Play - Give the majore product, when the following compounds are treated with...
Text Solution
|
Play - I. Which of the following solvents is the reaction faster? II. ...
Text Solution
|
Play - I. Give the products of the following: II. Which has faster ra...
Text Solution
|
Play - A Grignard regane t(A0 and a haloalkene (B) react together to give (C)...
04:59
|
Play - Cataylitc dehydrogention of methylcyclohexxane, obtained form petroleu...
Text Solution
|
Play - An organic compound (A) on analysis was found to contain C = 16.271%, ...
Text Solution
|
Play - Benzene reacts with CH(3)I in the presence of AlCl(3) to give compound...
04:29
|
Play - Two isomeric mononitro derivaters (B) and (C) are obtained by the nirt...
Text Solution
|
Play