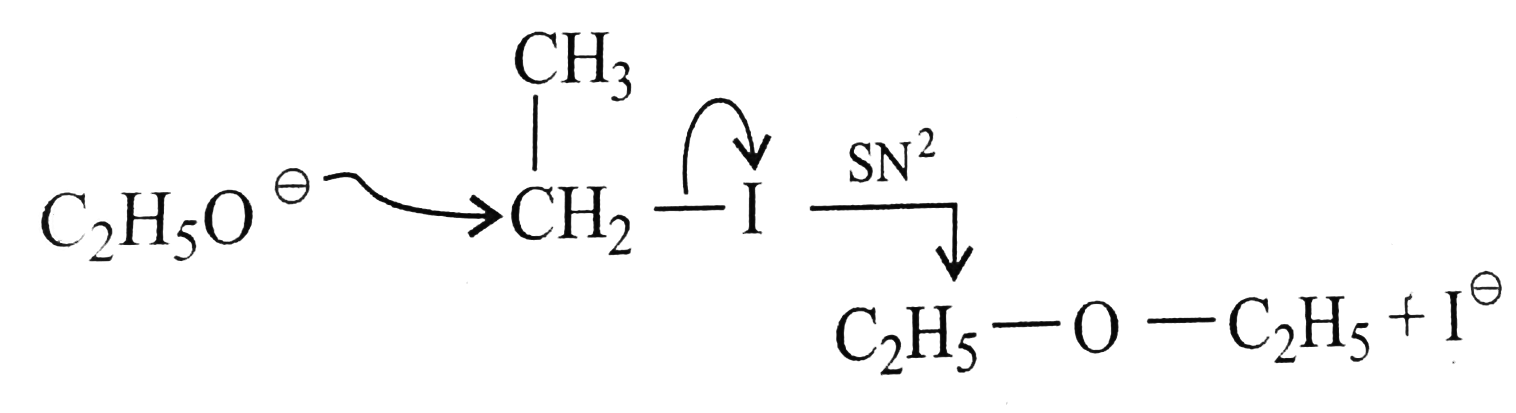
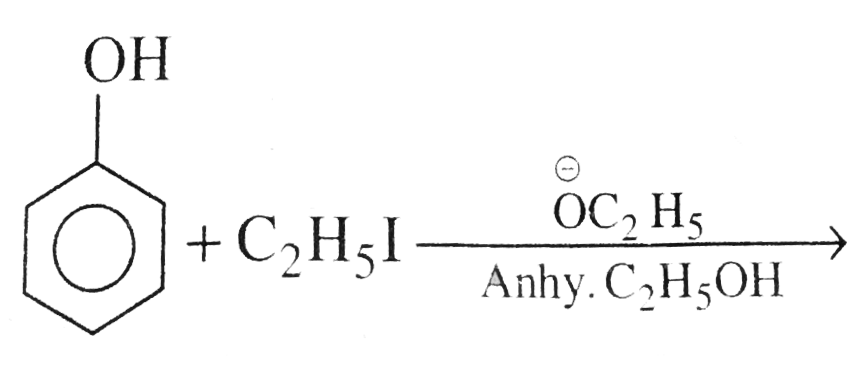Text Solution
Verified by Experts
The correct Answer is:
|
Topper's Solved these Questions
AROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Archives Mutiple Correct Answers Type|1 VideosView PlaylistAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Archives Mutiple Correct|5 VideosView PlaylistAROMATIC COMPOUNDS AND ALKYL AND ARYL HALIDES
CENGAGE CHEMISTRY|Exercise Archives Single Correct|21 VideosView PlaylistAPPENDIX INORGANIC VOLUME 2
CENGAGE CHEMISTRY|Exercise Short Answer Type|179 VideosView PlaylistBIOMOLECULES
CENGAGE CHEMISTRY|Exercise Exercises Archives (Analytical And Descriptive)|8 VideosView Playlist