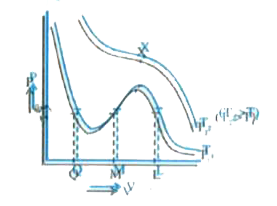
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE- PRACTICE SHEET (ADVANCED) (LINKED COMPREHENSION TYPE QUESTIONS) (PASSAGE- III)|3 VideosSOLUTIONS
AAKASH SERIES|Exercise Objective Exercise -4|50 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise PRACTICE SHEET (ADVANCED) (INTEGER TYPE QUESTION)|2 Videos