Text Solution
Verified by Experts
Topper's Solved these Questions
ACIDS, BASES, AND SALTS
ZEN PUBLICATION|Exercise ZEN ADDITOINAL QUESTIONS SECTIONS (Long Answer Type Questions)|21 VideosACIDS, BASES, AND SALTS
ZEN PUBLICATION|Exercise ZEN ADDITOINAL QUESTIONS SECTIONS (Short Answer Type Questions - I)|26 VideosCARBON AND ITS COMPOUNDS
ZEN PUBLICATION|Exercise ACTIVITY|2 Videos
Similar Questions
Explore conceptually related problems
ZEN PUBLICATION-ACIDS, BASES, AND SALTS-ZEN ADDITOINAL QUESTIONS SECTIONS (Short Answer Type Questions -II)
- State what happens when : (a) Gypsum is heated at 373 K (b) Blue c...
Text Solution
|
- "pH has a great importance in our daily life". Explain by giving three...
Text Solution
|
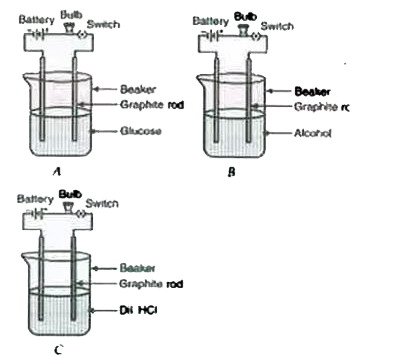
- Observe the following set up : (a) Which of the bulb glows ? (b...
Text Solution
|
- 2 ml of NaOH solution is added to a few pieces of granulated zinc meta...
Text Solution
|
- The pH of a salt used to make tasty and cripsy pakoras is 14. Identify...
Text Solution
|
- A student adds a spoon full of powdered sodium hydrogen carbonate to a...
Text Solution
|
- You have two solutions A and B. the pH of solution A is 6 and pH of so...
Text Solution
|
- How the following substances will dissociate to produce ions in their ...
Text Solution
|
- What is tooth enamel chemically ? State the condition when it starts c...
Text Solution
|
- Answer the following : What happens when crystals of washing soda ar...
Text Solution
|
- Name the change takes place.Which two industries are based on the use ...
Text Solution
|
- With the help of balanced chemical equation, state the reaction that t...
Text Solution
|
- How is plaster of Paris chemically different from Gypsum ? How can the...
Text Solution
|
- Define olfactory indicators.
Text Solution
|
- Choose the strong acids, from the following CH(3)COOH, H(2)SO(4), H(...
Text Solution
|
- Explain the action of dilute hydrochloric acid on the following with c...
Text Solution
|
- On passing excess carbon dioxide gas through lime water, it first turn...
Text Solution
|
- A white powder is added while baking bread and cakes to make them soft...
Text Solution
|
- What will be the action of the following substances on litmus paper ? ...
Text Solution
|
- Salt A commonly used in bakery products on heating gets converted into...
Text Solution
|