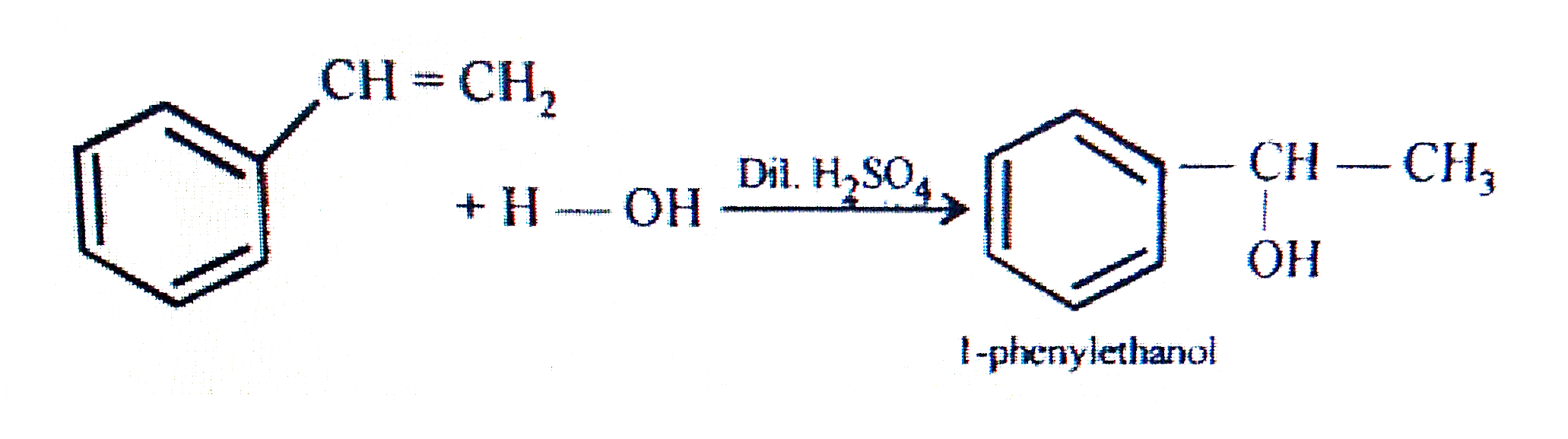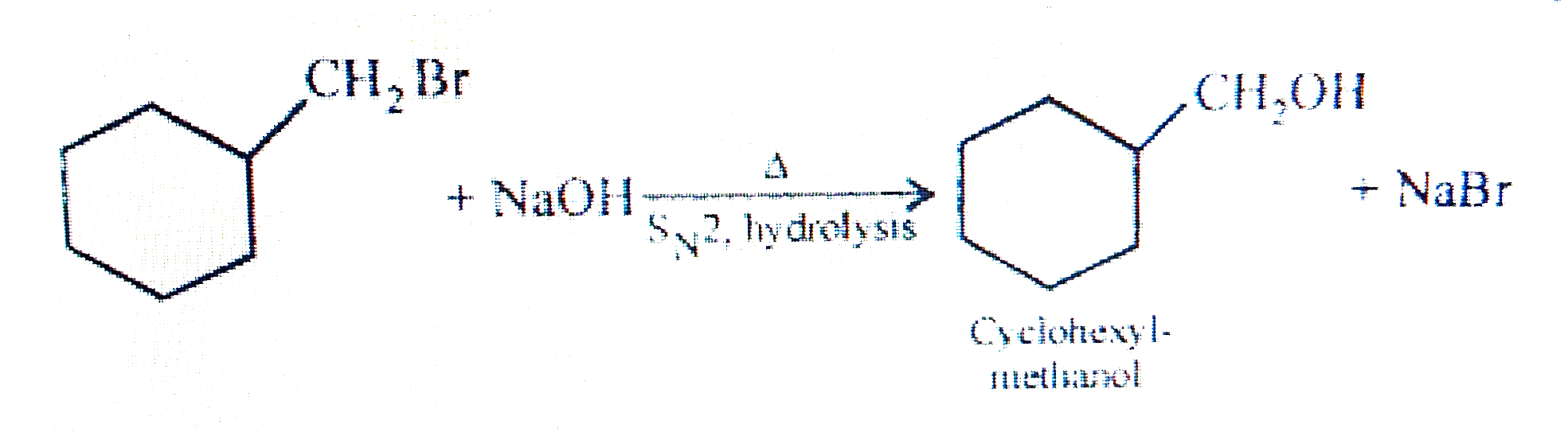Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Show how will you synthesie: i. 1-Phenylethanol form a suitable alk...
Text Solution
|
- Show how will you synthesie: i. 1-Phenylethanol form a suitable alkene...
Text Solution
|
- आप निम्न को कैसे संश्लेषित करेंगे? एक उपयुक्त ऐल्किल हैलाइड के उपयोग...
Text Solution
|
- SN^(1) reaction of alkyl halides leads to
Text Solution
|
- आप कैसे संश्लेषित करेंगे - (i) किसी उपयुक्त एल्कीन से 1-फिनॉलऐथेनोल,...
Text Solution
|
- How will you synthesise Cyclohexylmethanol using an alkyl halide by an...
Text Solution
|
- Show how will you synthesise: (i) 1-phenylethanol from a suitable al...
Text Solution
|
- प्रदर्शित कीजिये किस प्रकार आप संश्लेषित करेंगे- उपयुक्त एल्किल हैला...
Text Solution
|
- आप निम्नलिखित को कैसे संश्लेषित करेंगे, दर्शाइए - (i) एक उपयुक्त एल्...
Text Solution
|