Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
POLYMERS
AAKASH SERIES|Exercise Practice Sheet -5 (Single answer questions)|16 VideosPOLYMERS
AAKASH SERIES|Exercise Practice Sheet -5 (Linked comprehension type questions)|6 VideosPOLYMERS
AAKASH SERIES|Exercise Practice Sheet-4 (Match the following questions)|2 VideosPERIODIC TABLE
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|37 VideosPRACTICAL CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET-5 (Integer answer Type questions)|6 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-POLYMERS-Practice Sheet-4 (Integer answer Types Questions)
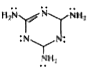
- The total number of lone pairs of electrons in melamine
Text Solution
|
- How many of the following are biodegradable co-polymers ? Nylon-2, N...
Text Solution
|
- How many of the following are biodegradable homopolymers ? PCL PHBV,...
Text Solution
|
- In the following polymer of monomer having how many -OH and -COOH grou...
Text Solution
|
- What are the oxidation states Al and Ti in the Zeigler - Natta catalys...
Text Solution
|
- In the polymer of Buna-S monomers are having how many pi-bonds ?
Text Solution
|