A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2 (Borax, Boric acids and Boron hydrides)|13 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 2 (Aluminum Chloride)|4 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -1 (Aluminium Chloride)|6 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II (PRACTICE SHEET ADVANCE)) (Integer Type Questions)|3 VideosELEMENTS OF CARBON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (Recent AIPMT/NEET Questions) |10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELEMENTS OF BORON FAMILY-OBJECTIVE EXERCISE - 2 (General introduction and properties)
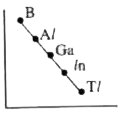
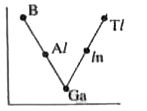
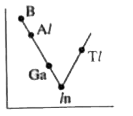
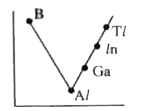
- Which one of the following correctly represents the variation of elect...
Text Solution
|
- IIIA group element with highest density is
Text Solution
|
- The ionisation energies from Ga to TI do not decrease due to
Text Solution
|
- Electronegativity is least for
Text Solution
|
- Which of the following is ionic
Text Solution
|
- Among the following most metallic element is
Text Solution
|
- The tendency of forming M("aq")^(3+) is highest for which IIIA group ...
Text Solution
|
- Least basic among the following is
Text Solution
|
- Al and Ga have nearly the same covalent radii, because of
Text Solution
|
- The maximum covalency of aluminium is 6 where as that of boron is '4' ...
Text Solution
|
- Which one of the following has the lowest melting point
Text Solution
|
- E(Al^(3+)//Al)^0 =- 1.66 V and E(Tl^(3+)//Tl)^0 = 1.26 V. Then which o...
Text Solution
|