A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - I) LEVEL - II (ADVANCED)|30 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise PRACTICE SHEET (EXERCISE - II) LEVEL - I (MAIN)|12 VideosELEMENTS OF BORON FAMILY
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE - II) LEVEL - II (ADVANCED)|27 VideosELECTRONIC EFFECTS AND REACTION INTERMEDIATES
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL - II (PRACTICE SHEET ADVANCE)) (Integer Type Questions)|3 VideosELEMENTS OF CARBON FAMILY
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (Recent AIPMT/NEET Questions) |10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-ELEMENTS OF BORON FAMILY-PRACTICE SHEET (EXERCISE - I) LEVEL - I (MAIN)
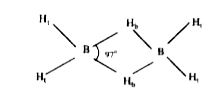
- The H-B-H bridged angle in diborane is
Text Solution
|
- The bonds not present in diborane is
Text Solution
|
- The main factor responsible for weak acidic nature of B - F bonds in B...
Text Solution
|
- B - F bond order of BF(3) is
Text Solution
|
- The two type of bonds present in B(2)H(6) are covalent and
Text Solution
|
- The green coloured borax bead obtained from copper salts is
Text Solution
|
- Borazole on strong heating gives
Text Solution
|
- Boron carbide, B(4)C is widely used
Text Solution
|
- Which one of the following is the correct statmenet
Text Solution
|
- Borax is coverted into crystalline boron by the following steps Bor...
Text Solution
|
- Which is not true about borax ?
Text Solution
|
- Indium and thallium of III A group have nearly similar atomic radii du...
Text Solution
|