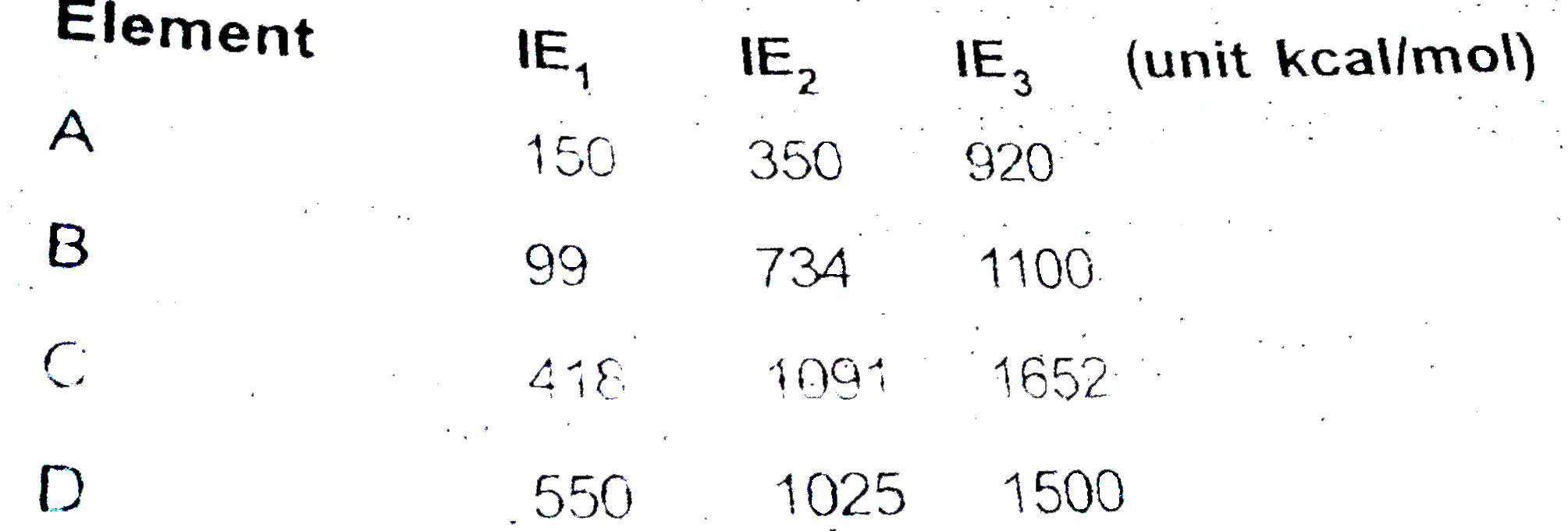A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
AAKASH INSTITUTE|Exercise Assignment (Section -E assertion-Reason Type Questions)|14 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
AAKASH INSTITUTE|Exercise Assignment (Section -F Matrix-Match Type Questions)|1 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
AAKASH INSTITUTE|Exercise Assignment (Section -C)|12 VideosCHEMISTRY IN EVERYDAY LIFE
AAKASH INSTITUTE|Exercise Assignment ( SECTION - A)|45 VideosCOORDINATION COMPOUNDS
AAKASH INSTITUTE|Exercise Assignment (Section-J Aakash Challenger Questions )|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH INSTITUTE-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-Assignment (Section -D Linked Compreshension Type Questions)
- Ionisation energy is the amount of energy required to remove the outer...
Text Solution
|
- Ionisation energy is the amount of energy required to remove the outer...
Text Solution
|
- Ionisation energy is the amount of energy required to remove the outer...
Text Solution
|
- Mulliken defined the electronegativity of an atom as the arithmetic me...
Text Solution
|
- Mulliken defined the electronegativity of an atom as the arithmetic me...
Text Solution
|
- Mulliken defined the electronegativity of an atom as the arithmetic me...
Text Solution
|