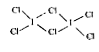
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- ICl(3) exist as dimer I(2) Cl(6) . In this molecule regarding this dim...
Text Solution
|
- Why boron halides do not exists as a dimer. While AlCl(3) exists as Al...
Text Solution
|
- AlCl(3) exists as dimer because
Text Solution
|
- BCl(3) does not exist as a dimer but BH(3) exists as B(2)H(6) because:
Text Solution
|
- Why boron halides do not exidising as dimers while AlCl(3) exists as A...
Text Solution
|
- BCl(3) does not exist as dimer but BH(3) exist as dimer (B(2)H(6)) bec...
Text Solution
|
- Dimer Al(2)Cl(6) is formed because
Text Solution
|
- Why boron halides do not exists as a dimer. While AlCl(3) exists as Al...
Text Solution
|
- BF(3) exists as discrete molecules but BH(3) exists as dimer. Exp...
Text Solution
|