Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET - 1 (Single or more than one option questions)|15 VideosNUCLEAR CHEMISTRY
AAKASH SERIES|Exercise PRACTICE SHEET - 1 (Linked Comprehension type questions )|6 VideosNUCLEAR CHEMISTRY
AAKASH SERIES|Exercise LECTURE SHEET (LEVEL- II) (EXERCISE - III) (Match the following questions )|3 VideosNOBLE GASES
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (Single more than one option questions)|5 VideosPERIODIC TABLE
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|37 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-NUCLEAR CHEMISTRY -LECTURE SHEET (LEVEL- II) (EXERCISE - IV) (Integer answer type Questions)
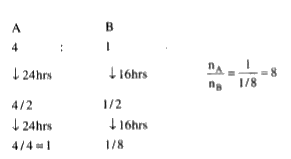
- A sample contains two radioactive substances A and B in the ratio of 4...
Text Solution
|
- A radioactive sample had an initial activity of 56 dpm . After 69.3 mi...
Text Solution
|
- Upon irradiating californium with neutrons , a scientist discovered a ...
Text Solution
|
- Na^(22) has half life of 2.68 years . It decays both by positron emiss...
Text Solution
|
- Iron -49 decay by positron emission with a half-life of 0.08 seconds ....
Text Solution
|
- No. of nuclei among the following which undergo radioactive decay (ba...
Text Solution
|
- Number of alpha - particles produced during the decay process of U - 2...
Text Solution
|
- In how many way among the following , a nucleus with high n/p ratio is...
Text Solution
|
- The periodic table consists of 18 groups. An isotope of copper, on bom...
Text Solution
|