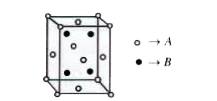
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - III)|5 VideosSOLID STATE
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - IV)|4 VideosSOLID STATE
AAKASH SERIES|Exercise LEVEL - II LECTURE SHEET (EXERCISE - I)|31 VideosREVISION EXERCISE
AAKASH SERIES|Exercise COMPLEX COMPOUNDS|47 VideosSOLIDS STATE
AAKASH SERIES|Exercise PRACTICE EXERCISE|63 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-SOLID STATE-LEVEL - II LECTURE SHEET (EXERCISE - II)
- If the molar mass AB is 100g mol^(-1) and 'a' is edge length then the ...
Text Solution
|
- The given unit cell belongs to
Text Solution
|
- The coordination number of 'B' will be
Text Solution
|
- A spinel is an important class of oxides consising of two types of met...
Text Solution
|
- A spinel is an important class of oxides consising of two types of met...
Text Solution
|
- A spinel is an important class of oxides consising of two types of met...
Text Solution
|