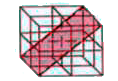
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
AAKASH SERIES|Exercise PRACTICE SHEET - 3|30 VideosSOLID STATE
AAKASH SERIES|Exercise PRACTICE SHEET - 4|30 VideosSOLID STATE
AAKASH SERIES|Exercise PRACTICE SHEET - 1|30 VideosREVISION EXERCISE
AAKASH SERIES|Exercise COMPLEX COMPOUNDS|47 VideosSOLIDS STATE
AAKASH SERIES|Exercise PRACTICE EXERCISE|63 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-SOLID STATE-PRACTICE SHEET - 2
- In a hypothetical solid C atoms are found to form cubical close packed...
Text Solution
|
- The intermediate compound LiAg crystallises in a cubic lattice in whic...
Text Solution
|
- AgI crystallises in the cubic close-packed zinc blend structure. Assum...
Text Solution
|
- For which of the following crystals would you expect the assumption of...
Text Solution
|
- In an atomic bcc, what fraction of edge is not covered by atoms ?
Text Solution
|
- The packing efficiency of a simple cubic crystal with an interstitial ...
Text Solution
|
- If all the atoms, on the shaded plane are removed then the molecular f...
Text Solution
|
- The formula of an oxide of iron is Fe(0.93)O(1.00). If the compound h...
Text Solution
|
- In a planar tetraatomic molecule, AB(3), A is at the centroid of the e...
Text Solution
|
- The distance between the two tetrahedral voids (along the body diagona...
Text Solution
|
- How many unit cell are present in 4.0 gm of crystal AB (formula mass o...
Text Solution
|
- Which of the following statement(s) is (are) correct for CaF(2),
Text Solution
|
- Which of the following informations are correct about CsCl?
Text Solution
|
- Select the correct statements
Text Solution
|
- Which of the follwing expression is correct for packing fraction of Na...
Text Solution
|
- Ferrous oxide has a cubic structure and eged length of the unit cell i...
Text Solution
|
- Cdo has NaCl structure with density 8.27 g/cc. If the ionic radius of ...
Text Solution
|
- O^(-2): "CCP" B^(3+) : "Half of octahedral Voids" A^(2+) : 1//8^("...
Text Solution
|
- O^(-2): "CCP" B^(3+) : "Half of octahedral Voids" A^(2+) : 1//8^("...
Text Solution
|
- O^(-2): "CCP" B^(3+) : "Half of octahedral Voids" A^(2+) : 1//8^("...
Text Solution
|