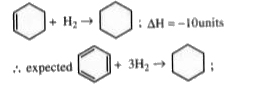Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-III) (LEVEL - I(MAIN) STRAIGHT OBJECTIVE TYPE QUESTIONS)|23 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-III) (LEVEL - II (ADVANCED) STARAIGHT OBJECTICE TYPE QUESTIONS)|12 VideosCHEMICAL THERMODYNAMICS
AAKASH SERIES|Exercise LECTURE SHEET (EXERCISE-II) (LEVEL - I(MAIN) MATRIX MATCHING TYPE QUESTIONS)|2 VideosCHEMICAL THERMODYANMICS
AAKASH SERIES|Exercise Questions For Descriptive Answers|28 VideosELECTRON MIGRATION EFFECTS
AAKASH SERIES|Exercise QUESTIONS FOR DESCRIPTIVE ANSWERS|10 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMICAL THERMODYNAMICS-LECTURE SHEET (EXERCISE-II) (LEVEL - I(MAIN) INTEGER TYPE QUESTIONS)
- What is the difference between heats of reaction at constant volume an...
Text Solution
|
- Heat of combustion of A(s) is -10 k"cal" mol^(-1) and that of B(s) is ...
Text Solution
|
- The bond dissociation energies of gaseous H(2), Cl(2) and HCl are 104,...
Text Solution
|
- If S + O(2) rarr SO(2), Delta H = -398.5 kJ SO(2) + (1)/(2)O(2) rarr...
Text Solution
|
- Heat of neutralisation of a polybasic acid by strong base is -54.8 kca...
Text Solution
|
- What is the resonance energy of Benzene in the same units?
Text Solution
|