Topper's Solved these Questions
TRANSPORT IN PLANTS
KUMAR PRAKASHAN|Exercise Section-C Definition/Explanation-terms/Location-Function|25 VideosTRANSPORT IN PLANTS
KUMAR PRAKASHAN|Exercise Section-D Textual Exercise|15 VideosTRANSPORT IN PLANTS
KUMAR PRAKASHAN|Exercise Questions from Module (Question Paper )|9 VideosTHE LIVING WORLD
KUMAR PRAKASHAN|Exercise OBJECTIVE SECTION (FILL IN BLANKS)|9 Videos
Similar Questions
Explore conceptually related problems
KUMAR PRAKASHAN-TRANSPORT IN PLANTS -Section-B Difference/Scientific Reasons
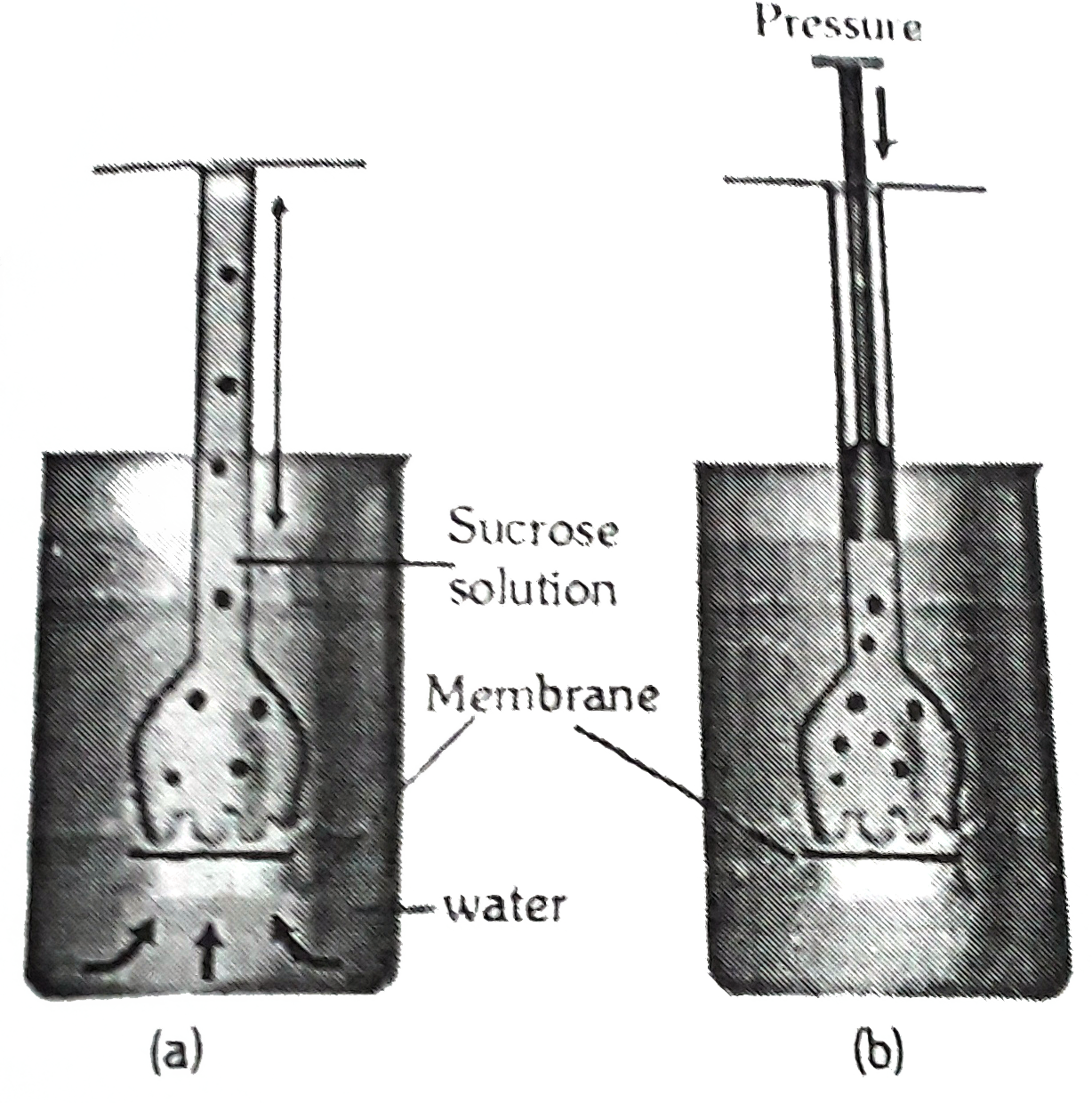
- Study the given figure carefully and select the true and false stateme...
Text Solution
|
- Plasmolysis is possible in plant cell while not possible in animal cel...
Text Solution
|
- Door-window of house swell in monsoon.
Text Solution
|
- Explain : transpiration is an unavoidable event.
Text Solution
|
- Water is life for plants.
Text Solution
|
- Ascent of sap is done by xylem tissue.
Text Solution
|