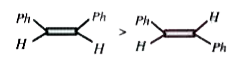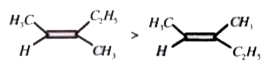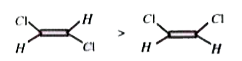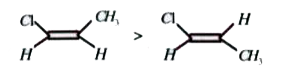A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-II (ADVANCED) Linked Comprehension Type Questions (Passage - II)|2 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-II (ADVANCED) Matrix Matching Type Questions|3 VideosISOMERISM
AAKASH SERIES|Exercise PRACTICE SHEET EXERCISE-II (Stereo Isomerism) LEVEL-II (ADVANCED) More than One correct answer Type Questions|5 VideosIONIC EQUILIBRIUM
AAKASH SERIES|Exercise ADDITIONAL PRACTICE EXERCISE (LEVEL -II PRACTICE SHEET (ADVANCED) (Integer Type Questions))|8 VideosPERIODIC CLASSIFICATION
AAKASH SERIES|Exercise OBJECTIVE EXERCISE - 3 (RECENT AIPMT/NEET QUESTIONS)|14 Videos
Similar Questions
Explore conceptually related problems