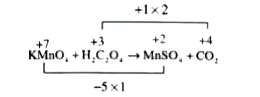Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY
AAKASH SERIES|Exercise Subjective Exericise -1 (Long answer questions)|1 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise Subjective Exericise -1 (Short answer questions)|13 VideosSTOICHIOMETRY
AAKASH SERIES|Exercise PROBLEM|92 VideosSTATES OF MATTER
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -3|19 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-STOICHIOMETRY-Illustrative Solved Examples
- Potassium permanganate oxidises oxalic acid in suphuric acid medium to...
Text Solution
|
- Sulphuric acid oxidises hydroiodic acid to iodine and forms hydrogen s...
Text Solution
|
- Manganese dioxide oxidises hydrochloric acid to chlorine and gives man...
Text Solution
|
- Chlorine reacts with cold dilute caustic soda to give sodium chloride,...
Text Solution
|
- Permanganate oxidises sulphite to sulphate in acidic solutions.
Text Solution
|
- Iodate oxidises chromic hydroxide and gives iodide and chromate in bas...
Text Solution
|
- Chromium metal in basic medium is oxidised in air to give chromic tetr...
Text Solution
|
- Write phosphorous reacts with aqueous caustic soda to give hypophosphi...
Text Solution
|
- Acetylene is oxidised by permanganate in acidic solutions to liberate ...
Text Solution
|
- Calculate the weight of calcium calcium carbonate required to produce ...
Text Solution
|
- What weight of magnesia is obtained by complete combustion of two gram...
Text Solution
|
- When 50 gm of a sample of sulphur was burnt in air 4% of the sample wa...
Text Solution
|
- What is the weight of calcium carbonate required for the production of...
Text Solution
|
- Calculate the volume of O(2) at STP required to burn completely 70 ml ...
Text Solution
|
- 2 L of hydrogen and 2.5 L of chlorine are allowed react in diffused li...
Text Solution
|
- 100 mL of phosphorus pentachloride is totally decomposed to its trichl...
Text Solution
|
- Methane undergoes slow atmospheric oxidation and produces carbonmonoxi...
Text Solution
|
- 50.0 kg of N(2)(g) and 10.0 kg of H(2)(g) are mixed to produce NH(2)(g...
Text Solution
|
- 100 mL of each acetylene and oxygen are mixed. The mixture is strongly...
Text Solution
|
- A jug contains 2L of milk. Calculate the volume of the milk in m^(3).
Text Solution
|