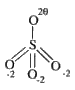A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
VIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 1 (Linked Comprehension type questions)|6 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 1 (Match the following questions)|2 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise LEVEL-II LECTURE SHEET (EXERCISE - IV Integer answer type Questions)|9 VideosVA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|51 VideosVII GROUP ELEMENTS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -4|55 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-VIA GROUP ELEMENTS-PRACTICE SHEET - 1 (Single or more than one option questions)
- The valency of sulphur in SO(4)^(2-) is :
Text Solution
|
- Which of the following bonds has the highest energy?
Text Solution
|
- H(2)S when passed through dil HNO(3) gives -
Text Solution
|
- The para magnetic nature of oxygen is best explained by
Text Solution
|
- Half - life of polonium is of
Text Solution
|
- Galena is a sulphide of
Text Solution
|
- In the Kipp's apparatus, the reaction gets stopped on closing the outl...
Text Solution
|
- The elements of group-16 which show negative oxidation state are 1)...
Text Solution
|
- On adding Na(2)S to sodium nitro prusside solution
Text Solution
|
- What is the correct relation ship between the pH values of isomolar so...
Text Solution
|
- H(2)S on incomplete combustion with oxygen forms mainly
Text Solution
|
- Which occur(s) free in nature?
Text Solution
|
- In SOCl(2) and SO(2)Cl(2)
Text Solution
|
- Which halide of sulphur undergoes disproportion ation in water?
Text Solution
|
- Which of the following statements are correct?
Text Solution
|
- The correct statement about sulphur hexa fluoride
Text Solution
|