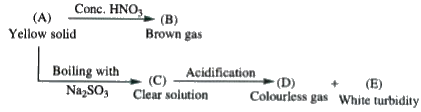A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
VIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 4 (Match the following questions)|2 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 4 (Integer answer type Questions)|2 VideosVIA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET - 4 (Single or more than one option questions)|16 VideosVA GROUP ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|51 VideosVII GROUP ELEMENTS
AAKASH SERIES|Exercise OBJECTIVE EXERCISE -4|55 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-VIA GROUP ELEMENTS-PRACTICE SHEET - 4 (Linked Comprehension type questions)
- Passage - I : Yellow solid 'A' is
Text Solution
|
- Passage - I : Brown gas 'B' is
Text Solution
|
- Passage - I : Solution 'C' is
Text Solution
|
- Passage - II : The oxidation state of 'Mn' in 'B' is
Text Solution
|
- Passage - II : During its reaction with Cl(2), 'X' acts as
Text Solution
|
- Blue colour of 'C' is due to the formation of
Text Solution
|