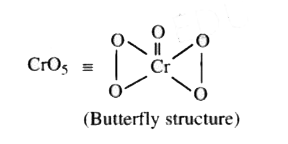
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-2 (SINGLE OR MORE THAN ONE OPTION QUESTIONS)|16 VideosD & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-2 (LINKED COMPREHENSION TYPE QUESTIONS)|6 VideosD & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-1 (MATCH THE FOLLOWING QUESTIONS)|3 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise PRACTICE EXERCISE|45 VideosD - BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|50 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-D & F BLOCK ELEMENTS-PRACTICE SHEET-1 (INTEGER ANSWER TYPE QUESTIONS)
- The oxidation state of CrO(3)
Text Solution
|
- The number of transition metals in the alloy Alnico is
Text Solution
|
- How many of the following the second element has higher density than t...
Text Solution
|
- The oxidation number of the metal in Zeisel's salt is
Text Solution
|
- The number of unpaired in the central metal ion of brown ring complex ...
Text Solution
|