A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-3 (MATCH THE FOLLOWING QUESTIONS)|2 VideosD & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-3 (INTERGER ANSWER TYPE QUESTIONS)|6 VideosD & F BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE SHEET-3 (SINGLE OR MORE THAN ONS OPTION QUESTIONS)|16 VideosCOMPLEX COMPOUNDS
AAKASH SERIES|Exercise PRACTICE EXERCISE|45 VideosD - BLOCK ELEMENTS
AAKASH SERIES|Exercise PRACTICE EXERCISE|50 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-D & F BLOCK ELEMENTS-PRACTICE SHEET-3 (LINKED COMPREHENSION TYPE QUESTIONS)
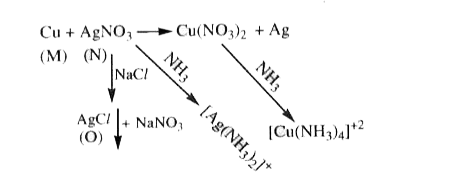
- When a metal rod M is dipped into an aqueous colourless concentreted s...
Text Solution
|
- When a metal rod M is dipped into an aqueous colourless concentreted s...
Text Solution
|
- The final solution contains
Text Solution
|
- A colourless inorganic compound(A) is soluble in water, alcohols, amin...
Text Solution
|
- A colourless inorganic compound(A) is soluble in water, alcohols, amin...
Text Solution
|
- A colourless inorganic compound(A) is soluble in water, alcohols, amin...
Text Solution
|