A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
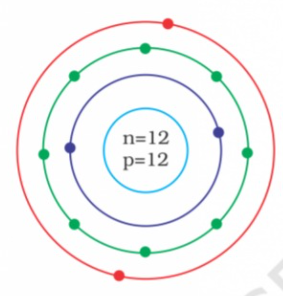
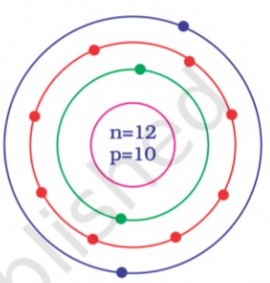
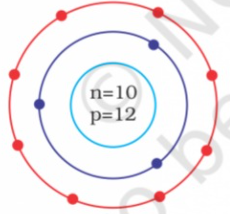
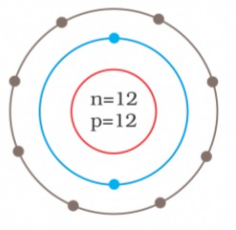
- Identify the Mg^(2+) ion from the figure where, n and p represent the ...
Text Solution
|
- m(P) and m(n) are masses of proton and neutron respectively. An elemen...
Text Solution
|
- If the nuclear force between two protons, two neutrons and between pro...
Text Solution
|
- Identify the Mg^(2+) ion from the figure where, n and p represent the ...
Text Solution
|
- m(p) and m(n) are masses of proton and neutron respectively. An elemen...
Text Solution
|
- M(p) तथा M(n) प्रोटॉन तथा न्यूट्रॉन द्रव्यमान दर्शाते हैं , एक नाभि...
Text Solution
|
- M(n) तथा M(p) क्रमशः न्यूट्रॉन तथा प्रोटॉन के द्रव्यमानों को प्रदर्शित...
Text Solution
|
- M(n) and M(p) represent mass of neutron and proton respectively. If an...
Text Solution
|
- Identify the Mg^(2+) ion from the figure where, n and p represent the ...
Text Solution
|