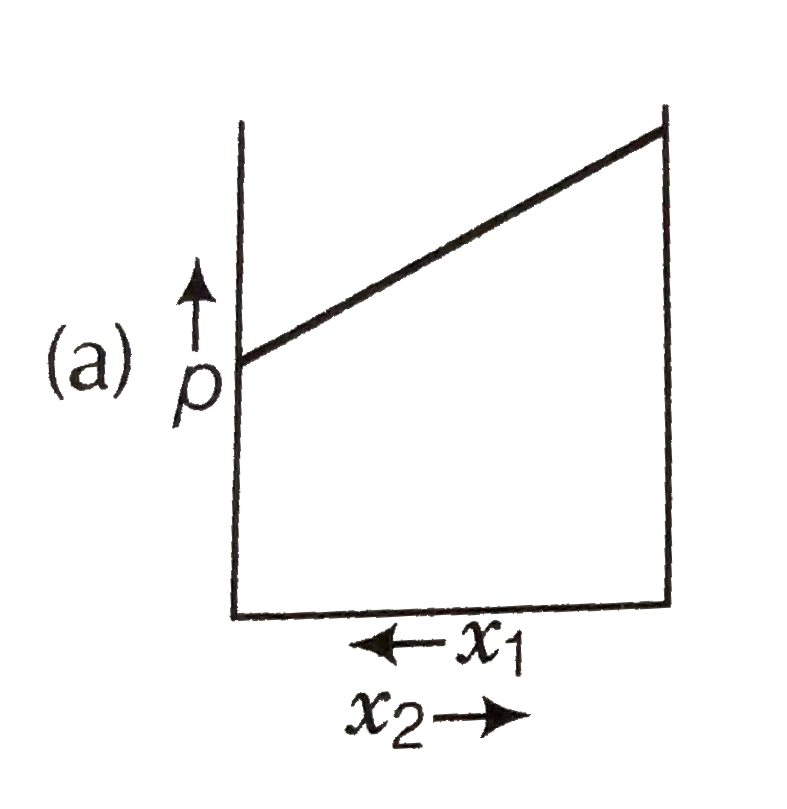
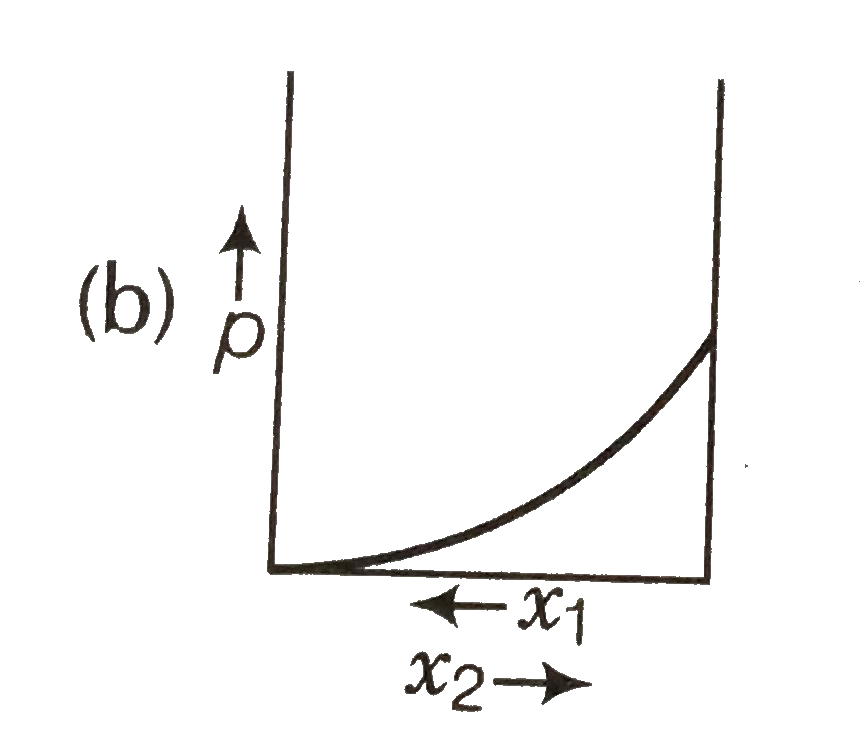
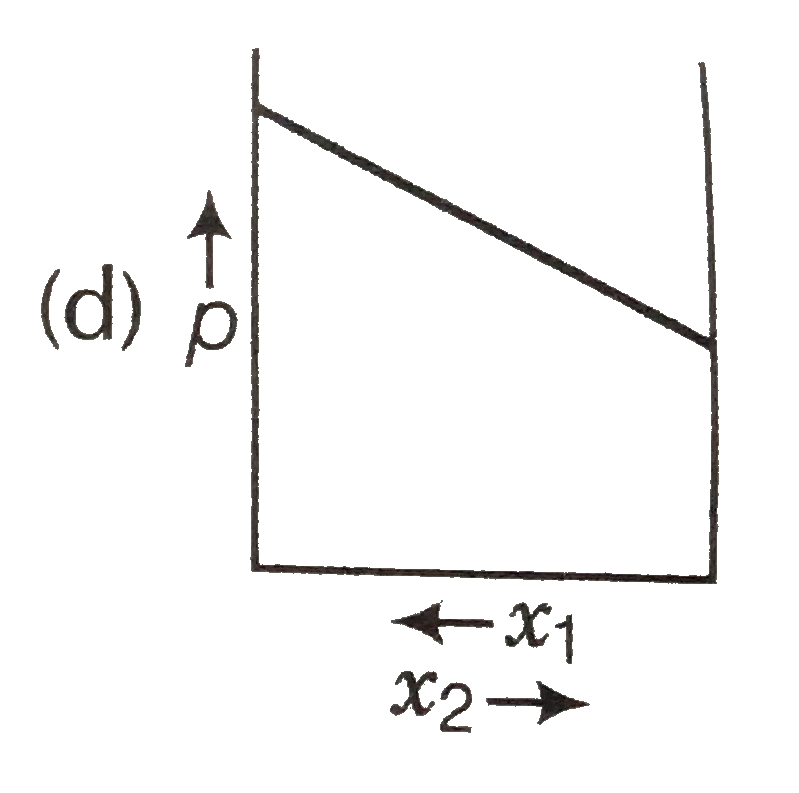
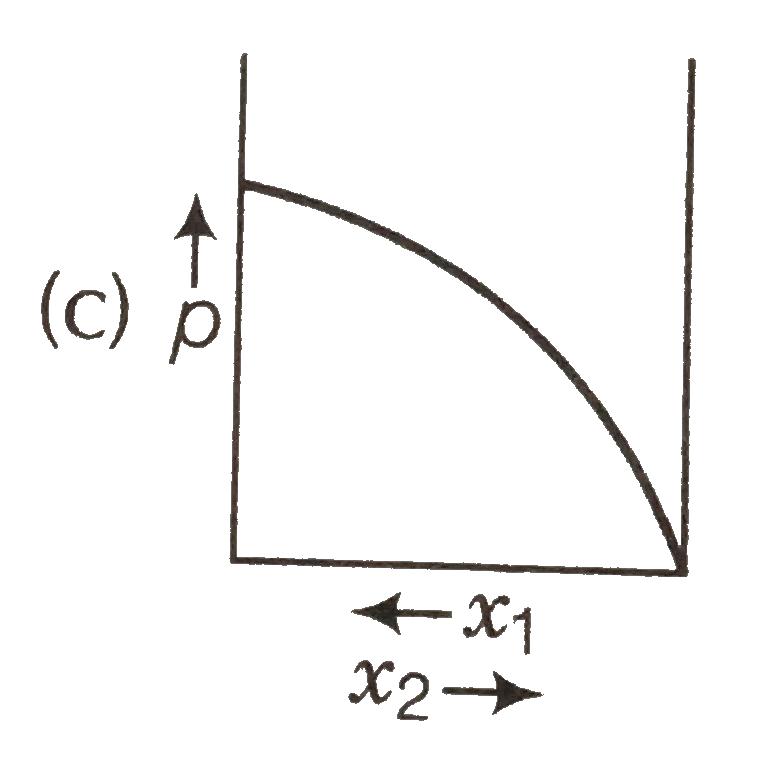A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER TYPE QUESTIONS|8 VideosSOLUTIONS
NCERT EXEMPLAR ENGLISH|Exercise LONG ANSWER TYPE QUESTIONS|8 VideosSOLID STATE
NCERT EXEMPLAR ENGLISH|Exercise long answer type questions|4 VideosSURFACE CHEMISTRY
NCERT EXEMPLAR ENGLISH|Exercise Long Answer Type Questions|4 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR ENGLISH-SOLUTIONS -MULTIPLE CHOICE QUESTIONS (MORE THAN ONE OPTIONS
- Which of the following binary mixture will have same composition in li...
Text Solution
|
- In istonic solutions………..
Text Solution
|
- For a binary ideal liquid solution, the variation total vapour pressur...
Text Solution
|
- Colligative properties are observed when……..
Text Solution
|
- Components of a binarey mixture of two liquids A and B were being sepa...
Text Solution
|
- Explain in why on addition of 1 moe of NaCl to 1L of water, the boilin...
Text Solution
|
- Explain the solubility rule "like dissolves like" in terms of intermol...
Text Solution
|
- Concentration terms such as mass percentage, ppm, mole fraciton and mo...
Text Solution
|
- What is the significance of Hanry's law constant K(H) ?
Text Solution
|
- why are the aquatic species more comofortable in cold water in compari...
Text Solution
|
- (a) Explain the following phenomena with the help of Henry's law. (i...
Text Solution
|
- Why is the vapous pressure of an aqueous solution of gulucose lower th...
Text Solution
|
- How does sprinking of salt help in clearing the snow covered roads in ...
Text Solution
|
- What is "esmipermeble membrane"?
Text Solution
|
- Give an example of a material used for makin gsemipermeable membrance ...
Text Solution
|
- Match the following columns
Text Solution
|
Text Solution
|
- Calculate the equilibrium constant for the reaction Fe(s)+Cd2+(aq)⇔Fe2...
Text Solution
|
- Match the following columns
Text Solution
|
- Assertion (A) Molarity of a solution in liquid state changes with temp...
Text Solution
|