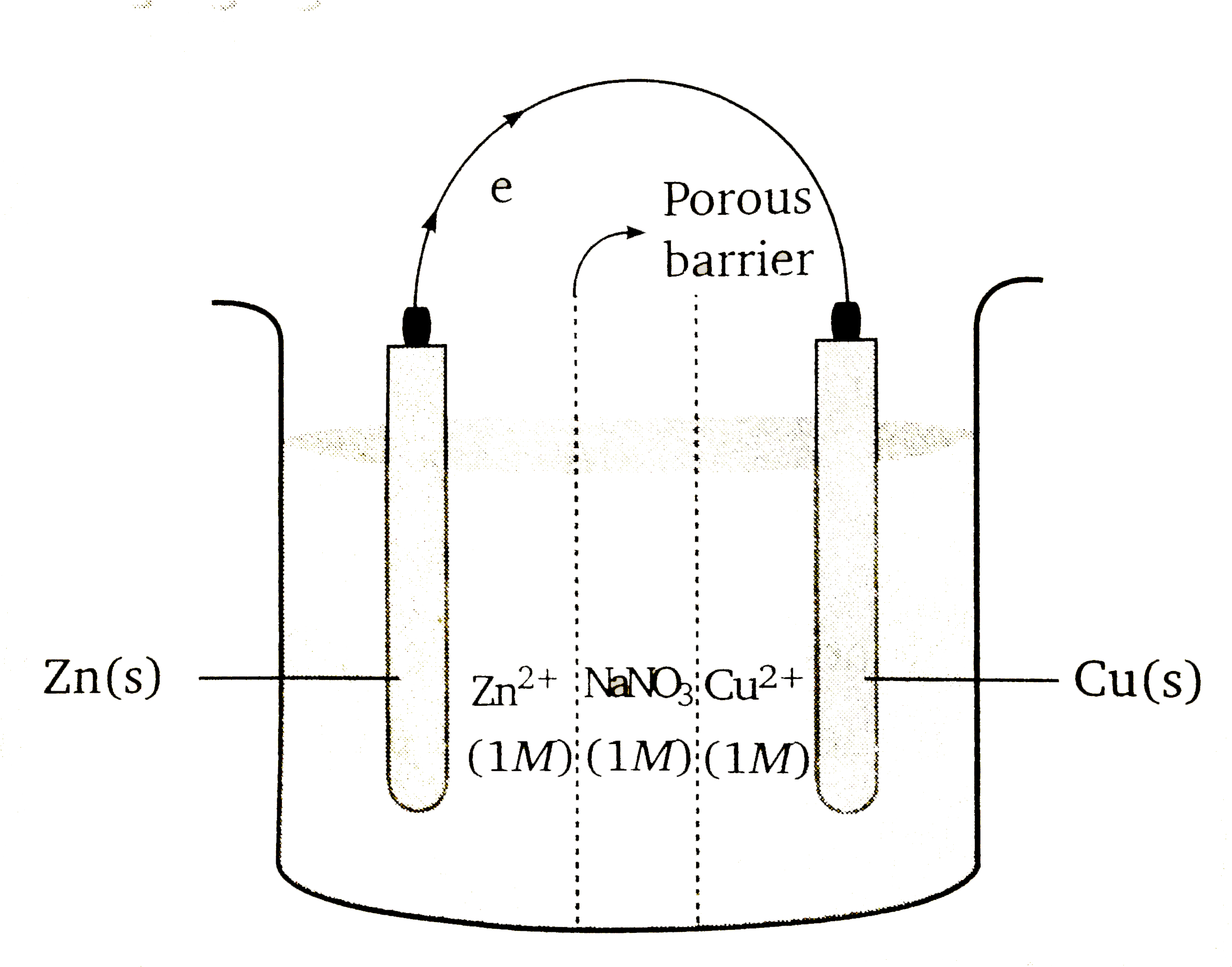A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
NARENDRA AWASTHI ENGLISH|Exercise Match the column|6 VideosELECTROCHEMISTRY
NARENDRA AWASTHI ENGLISH|Exercise Assertion Reason type question|15 VideosELECTROCHEMISTRY
NARENDRA AWASTHI ENGLISH|Exercise LEVEL-2|28 VideosDILUTE SOLUTION
NARENDRA AWASTHI ENGLISH|Exercise leval-03|23 VideosGASEOUS STATE
NARENDRA AWASTHI ENGLISH|Exercise Subjective problems|15 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI ENGLISH-ELECTROCHEMISTRY-LEVEL-3
- A Galvanic cell consits of three compartment as shown in figure. The f...
Text Solution
|
- A Galvanic cell consits of three compartment as shown in figure. The f...
Text Solution
|
- A Galvanic cell consits of three compartment as shown in figure. The f...
Text Solution
|
- The cell potential (E(cell)) of a reaction is related as /\G=-nF E(ce...
Text Solution
|
- At 300 k, /\ Hfor the reaction Zn(s)+AgCl(s)toZnCl2(aq)+2Ag(s) is -...
Text Solution
|
- At 300 k, /\ Hfor the reaction Zn(s)+AgCl(s)toZnCl2(aq)+2Ag(s) is -...
Text Solution
|
- The molar conductivity of 0.04 M solution of MgCl2 is 200 Scm^2mol^(-1...
Text Solution
|
- The molar conductivity of 0.04 M solution of MgCl2 is 200 Scm^2mol^(-1...
Text Solution
|
- In a hydrogen oxyge fuel cell, electricity is produced. In this proces...
Text Solution
|
- In a hydrogen oxyge fuel cell, electricity is produced. In this proces...
Text Solution
|
- In a hydrogen oxyge fuel cell, electricity is produced. In this proces...
Text Solution
|
- A saturated solution in AgX(K(sp)=3xx10^(-12)) and AgY(K(sp)=10^(-12)...
Text Solution
|
- A saturated solution in AgX(K(sp)=3xx10^(-12)) and AgY(K(sp)=10^(-12)...
Text Solution
|
- If the e.m.f of a galvanic cell is negative, it implies that:
Text Solution
|
- Select correct statement(s) about electrolysis:
Text Solution
|
- If the half-cell reaction A+e^(-)toA^(-) has a large negative reductio...
Text Solution
|
- Which of the following statements is correct? If E(Cu^(2+)|Cu)^(@)=0.3...
Text Solution
|
- The oxidation potential of hydrogen half-cell will be negative if:
Text Solution
|
- which of the following arrangement will procedure oxygen at anode dur...
Text Solution
|
- When an aqueous concentrated solution of lithium chloride is electroly...
Text Solution
|