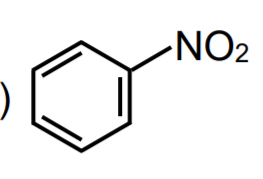
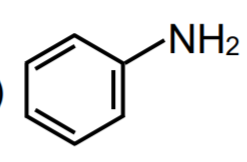
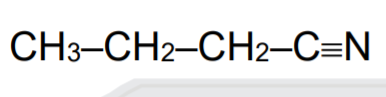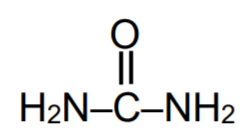A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Kjeldahl method cannot be used for :
Text Solution
|
- In DNA and RNA, nitrogen atom is present in the ring system. Can Kjeld...
Text Solution
|
- Kjeldahl method for estimation of nitrogen is not applicable to
Text Solution
|
- In DNA and RNA, nitrogen atom is present in the ring system. Can Kjeld...
Text Solution
|
- Kjeldahl method cannot be used for :
Text Solution
|
- Kjeldahl's method cannot be used for the estimation of nitrogen in
Text Solution
|
- In Kjeldahl's method, the nitrogen present in the organics compund is ...
Text Solution
|
- The catalyst used in Kjeldahl's method for the estimation of nitrogen ...
Text Solution
|
- In the first step Kjeldahl method for estimation of nitrogen in organi...
Text Solution
|