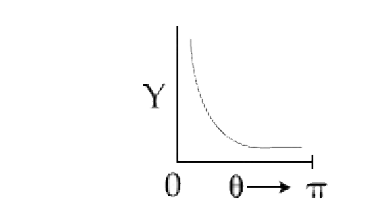
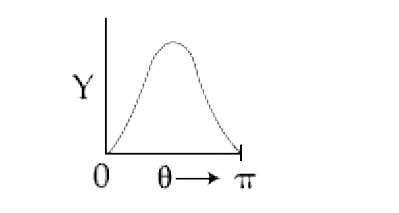
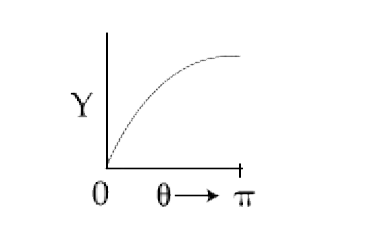
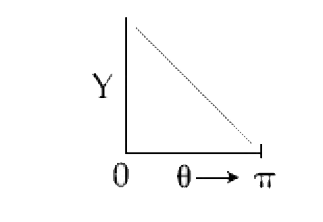
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
JEE MAINS PREVIOUS YEAR ENGLISH-JEE MAIN-All Questions
- The sum of two forces vecP and vecQ is vecR such that |vecR|=|vecP|. T...
Text Solution
|
- A battery of unknown emf connected to a potentiometer has balancing le...
Text Solution
|
- Number of the alpha- particle deflected in Rutherford's alpha -scatter...
Text Solution
|
- Find the co-ordinates of centre of mass of the lamina shown in figure
Text Solution
|
- When proton of KE = 1.0 MeV moving in South to North direction enters ...
Text Solution
|
- Boolem relation at the output stage-Y of the Followng circuit is:
Text Solution
|
- Which graph correctly represents variation between relaxation time (t)...
Text Solution
|
- Two photons of energy 4eV and 4.5 eV incident on two metals A and B re...
Text Solution
|
- If relative permittivity and relative permeability of a medium are 3 a...
Text Solution
|
- A cylinder of height 1m is floating in water at 0^@C with 20cm height ...
Text Solution
|
- A thermodynamic cycle xyzx is shown on a V-T diagram. The P-V di...
Text Solution
|
- A telescope has magnification 5 and length of tube 60cm then the focal...
Text Solution
|
- A particles of mass m is fixed to one end of a light spring of force c...
Text Solution
|
- The dimension of stooping potential V(0) in photoelectric effect In u...
Text Solution
|
- In fiinding the electric field using Gauses law the formula |vec(E)|=(...
Text Solution
|
- 3 charges are placed in a circle of radius d as shown in figure. Find ...
Text Solution
|
- There is a potentiometer wire of length 1200 cm and 60 mA current is f...
Text Solution
|
- At time t=0 magnetic Field of 1000 Gausses is passing perpendicularly ...
Text Solution
|
- Consider a solid sphere oF radius R and mass density rho(r)=rho(0)(1-(...
Text Solution
|
- Effective capacitance of parallel combination of two capacitors C(1) a...
Text Solution
|