d orbital : d orbital exist in energy level n=3 ,4 5… but in n=1 ,2 orbital d orbital is not exist so 1 and 2 d not exist
not of d orbitals no of orbits = (2l + 1) for d orbital l=2 so no of d orbitals are five (5) so d sub shell has five orbitals
No of d orbitals : for d orbitals l=2 and magnetic quantum number `m_(1) = - 2 ,-1 ,0, +1`
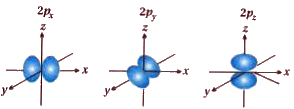
Shape of d orbitals : four d orbitals have same shape with four lobes So `d_(xy) d_(yz)` and `d_(zx)` have lobes in their xy, yz , zx plane which for `d_(x)^(2) ` the lobes are on the x and y axes.
Shape of `d_(x )` orbital is different form other in which two lobes around 2 axis .
Energy of d orbital : In any one sub shell the energy of all five d orbitals is same and equivalent their shape and size also same it the principal quantum number increase and size and energy also increase and size energy also increase 3d `lt 4d lt 5d`
Radial node and Angular node : where the lobes combine there electron density is zero in d orbitals angular node is l and radial node is (n-1-1)
for 3d orbital node (n-1) =3 -2 -1=0
Angular node 1=2
For 3d orbital total nodes = n- 1=3 -1=2 nodes.
