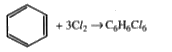Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMISTRY IN EVERYDAY LIFE
AAKASH SERIES|Exercise PRACTICE SHEET - 2 (PRACTICE SHEET -2 (MATCH THE FOLLOWING QUESTIONS))|2 VideosCHEMISTRY IN EVERY DAY LIFE
AAKASH SERIES|Exercise PRACTICE EXERCISE|29 VideosCO-ORDINATION COMPOUNDS
AAKASH SERIES|Exercise PRACTICE SHEET - 5 ( Linked Comprehension type questions Passage : I I:)|3 Videos
Similar Questions
Explore conceptually related problems
AAKASH SERIES-CHEMISTRY IN EVERYDAY LIFE -PRACTICE SHEET - 2 (PRACTICE SHEET -2 (INTEGER ANSWER TYPE QUESTIONS))
- One mole Benzene in presence of light on reaction with 'x' moles of Cl...
Text Solution
|
- How many functional groups are present in Norethindrone.
Text Solution
|
- Number of Amino acids present in Aspartame ?
Text Solution
|
- Number of Nitrogen atoms in histidine ?
Text Solution
|
- Mg(OH)2 , Al(OH)3 , omeprazole, Lansoprazole , sodium benzoate , sodiu...
Text Solution
|
- Testostrom, progesteron , norethindrone , ethynylestradial. Among them...
Text Solution
|
- Number of fused six membered rings in sterol ?
Text Solution
|